D.A. Davey
Corresponding Author: Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Western Cape, South Africa 7925, e-mail profdad@eject.co.za, Tel +27 21 712 1314
Abstract
Abstract: Alzheimer’s disease (AD) and cerebrovascular disease (CVD) frequently co-exist and CVD acts additionally and synergistically with AD in ageing–related impairment of cognitive function and dementia. A significant number of men and women with normal cognition at the time of death have the neurodegenerative and cerebrovascular changes of AD and CVD and are regarded as having high cognitive reserve or cognitive resilience. Many measures used to prevent and treat cardiovascular disease, decrease the incidence, or delay the onset of ageing-related cognitive impairment and dementia. Ageing-related cognitive impairment and dementia are increased by adverse psycho-social factors and can be prevented or mitigated by appropriate psycho-social measures. There is now more than sufficient evidence to implement, as a matter of urgency, personal health and life-style measures and public health initiatives in the endeavor to prevent, postpone or ameliorate ageing-related cognitive impairment and dementia and to decrease its burden world-wide.
Key words: Alzheimer’s disease, cognitive impairment, cerebrovascular disease, dementia, prevention.
Nomenclature
Alois Alzheimer in 1906 described a “peculiar severe disease process of the cerebral cortex” with “miliary foci” (β-amyloid plaques) and “fibrils” (neurofibrillary tangles) in a patient with dementia praecox and the condition was named “Alzheimer’s Disease” (1). The term “Alzheimer’s Disease” is currently used in several different senses:
(a) specifically, by neurologists, psychiatrists and others to mean the form of neurodegeneration characterized by β-amyloid plaques and neurofibrillary tangles in the brain as described by Alzheimer. The term “vascular dementia” (VaD) is used for dementia attributed to cerebrovascular disease
(b) loosely, to include all forms of ageing-related cognitive impairment and dementia with varying cerebral pathologies
(c) generally, in non-medical circles instead of the word “dementia”.
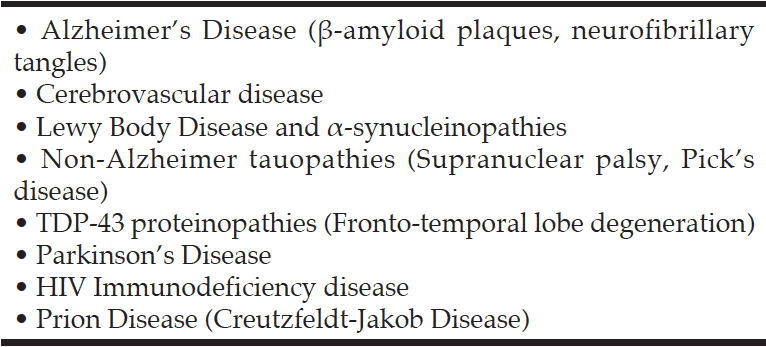
The different uses of the term “Alzheimer’s Disease” have led to misunderstanding and the meaning may only be clear from the context. Alzheimer’s disease (AD) as first described by Alzheimer is but one of several causes of Ageing-Related Cognitive Impairment and Dementia (ARCID) (Table 1). The commonest are AD, cerebrovascular disease (CVD) and Lewy Body Disease (LBD) which frequently co-exist .It has been proposed that ARCID and dementia should be regarded as a syndrome i.e. a complex of symptoms with multiple causes, similar to other chronic diseases (2).
The purpose of this review is to substantiate the evidence that:
(A) AD and CVD are commonly associated and act additively and synergistically in ARCID
(B) Many risk factors for ARCID and measures that may prevent or postpone its development are very similar to the risk factors and measures to prevent and treat cardiovascular and cerebrovascular disease.
(C) Adverse psycho-social factors are significant risk factors for ARCID and psycho-social measures that increase cognitive reserve and resilience may prevent, delay the onset or ameliorate ARCID
(D) In the current absence of effective disease-modifying treatments, primary prevention combining all possible protective measures is the best hope to prevent, delay the onset and ameliorate ARCID
Association of Alzheimer’s disease and cerebrovascular disease
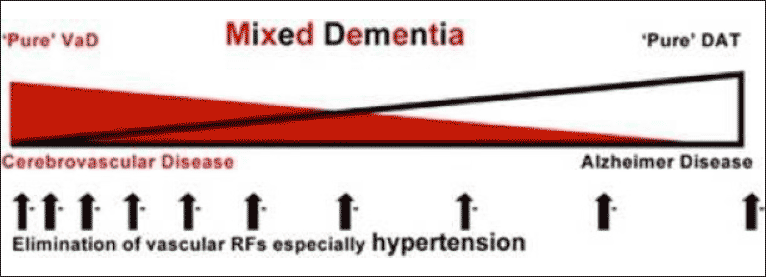
The cerebral pathology in men and women with dementia and of those with normal cognition at the time of death has been investigated in at least four major post-mortem studies: the Religious Orders Study and Rush Memory and Aging Project, the Medical Research Council Cognitive and Ageing Study, the Vienna Trans-Danube Aging Study, and The National Alzheimer’s Coordinating Centre USA Study (3-6). The main conclusions were very similar in all four studies, namely that the changes of AD and CVD (a) frequently co-exist in late-onset dementia (b) overlap to varying degrees and have additive and synergistic effects on cognitive decline (c) are sometimes found in persons with normal cognition at the time of death (7). The neurodegenerative and cerebrovascular changes associated with dementia form a spectrum from “pure” AD to “pure” CVD and most commonly are combined and result in “mixed dementia” (8) Fig1. The separation of AD as described by Alzheimer, and “vascular dementia” has been claimed to be a false dichotomy (8).
Cognitive reserve and cognitive resilience
A significant proportion of men and women with normal cognition at the time of death have the neurodegenerative and cerebrovascular changes of the brain associated with AD, CVD and dementia. The discordance between neuropathology and lack of cognitive impairment constitutes prima facie evidence for the role of some type of brain, neural or cognitive reserve (9). The absence of impaired cognition and dementia in such cases has been ascribed to high cognitive reserve and cognitive resilience. Cognitive reserve” implies high cognitive ability from early in life and its maintenance in mid and later life with the consequent prevention or postponement of ARCID (10). “Cognitive resilience” refers to the prevention or delay of ARCID in spite of the development of the pathological changes of AD, CVD and LBD.
Risk factors for ageing-related cognitive impairment and dementia
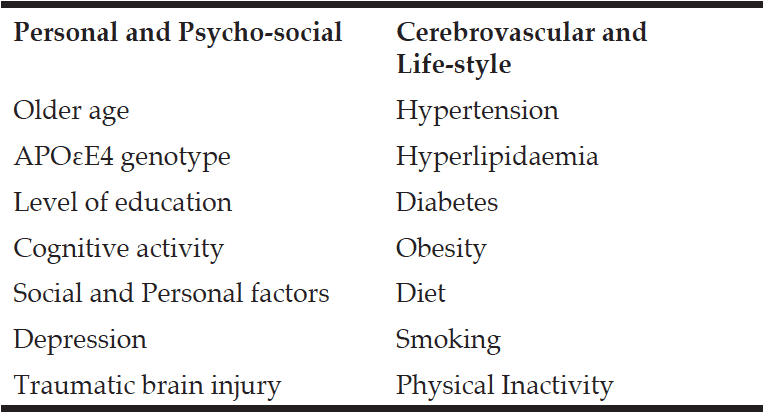
Ageing-related cognitive impairment and dementia has been associated with a large number of risk factors. A recent extensive meta-analysis of 323 papers including 93 factors considered suitable for epidemiological analysis, identified nine potentially modifiable risk factor; type-2 diabetes, obesity, hypertension, homocystinaemia, frailty, depression, current smoking, carotid artery narrowing, low educational achievement (11). The calculated population attributable risk combining all nine factors was 0.66 and it was claimed that two third of AD cases could be explained by these factors. In another study, potentially modifiable risk factors have been estimated to be present in approximately 50% of individuals with AD in the USA and worldwide (12). The seven modifiable risk factors included in these estimates were midlife hypertension, midlife obesity, diabetes mellitus, physical inactivity, smoking, depression and low education. The estimates do not take into account the non-independence of risk factors and the combined population-attributable risk factors have been estimated to be about 30% in the USA and Europe (13). Risk Factors can be divided into (a) Personal and Psycho-Social and (b) Cerebrovascular and Lifestyle (Table 2).
Personal factors
Personal factors including age, family history and the presence of the lipoprotein APOEε4 allele, are not modifiable but their effects can be mitigated or postponed by favourable environmental factors. Age is the most important factor determining the incidence and prevalence of cognitive impairment and dementia; the incidence of all-cause dementia increases exponentially from about 5/1,000 person-years in the 65-69 years age group to about 85/1,000 person-years in the age 90+ years (14). The most common genetic risk factor is the ε4 allele of the lipoprotein APOE4 and the APOEε4 allele has been estimated to increase the risk of AD about 3 times in heterozygotes and 15 times in homozygotes (15).
Psycho-social factors
Psycho-social factors often play an important part in ARCID and measures that increase cognitive reserve and cognitive resilience may be of considerable benefit in preventing, delaying or ameliorating ARCID. In an analysis of more than 20 studies involving 29,000 individuals followed for a median of 7.1 years, higher brain reserve was associated with a lower risk for incident dementia OR 0.54 (0.49–0.59) (10). The psycho-social factors that have been studied include, level of education, continuing cognitive activity and cognitive interventions, social and personality factors, depression and traumatic injury.
Level of education
The relative risks for low versus high education in a meta-analysis of 13 cohort and 6 case-control studies were, for AD 1.80 (1.43–2.27), for non-AD 1.32(0.92–1.88) and for all dementias 1.59(1.26–2.01) (16). In a meta-analysis of 31 studies with incident AD the pooled relative risk for lower education was RR 1.99(1.30–3.04)(17). In an analysis of 22 longitudinal studies including 21,456 individuals and 1,733 cases of dementia, the risk of dementia was lower for those with higher education OR 0.53 (0.45–0.62) (17). Low level of education is one of the biggest contributors to the high prevalence of AD world-wide (12).
Continued Cognitive Activity and Cognitive Interventions
A systematic review of 22 cohort studies including 29,000 individuals concluded that complex patterns of mental activity in early and mid-life was associated with a significant reduction in the incidence of dementia in later life RR 0.54(0.49–0.59) (10). In the Rush Memory Project frequent participation in cognitive stimulating activities was associated with less rapid decline in cognitive function and a lower incidence of AD, HR 0.58 (0.44–0.77) after controlling for a low baseline cognitive function, past cognitive activity, socioeconomic status and current social and physical activity (18). A Cochrane review in 2011 concluded that cognitive training interventions significantly improved immediate and delayed recall in healthy older adults and that more studies in other cognitive domains were necessary (19).
Social and Personality Factors
Social isolation and loneliness increase cognitive decline and the risk of late-life dementia (20, 21). Conscientiousness and purpose in life have been associated with a reduced risk of ARCID (22, 23). In the MRC-CFAS Study a combined Cognitive Lifestyle Score (CLS) based on educational attainment, occupational complexity and social engagement found that those who maintained a high CLS throughout life had a 40% reduced risk of developing dementia (24).
Depression
Depression may be a cause or consequence of cognitive impairment and dementia. A systematic review and meta-analysis of 20 studies including1,020,172 individuals found that history of depression increased risk of developing AD with a pooled OR of 2.03(1.73–2.38) for case control studies and of 1.90 (1.55–2.33) for cohort studies (25).
Traumatic brain Injury
Moderate and severe traumatic brain injury increases the risk of cognitive decline and is estimated to increase the risk of dementia in later life two to three fold (26). There is an increased risk of cognitive impairment and later onset of dementia in military veterans who have suffered brain injuries and in those involved in sports such as boxing and football of all forms, particularly in players who have experienced multiple concussions (27).
Cerebrovascular and life-style factors
Many cerebrovascular and lifestyle factors that predispose to ageing-related MCI and dementia are potentially preventable or modifiable (7). Measures that may prevent CVD are similar to those that prevent cardiovascular disease and include active treatment of hypertension, hyperlipidaemia and diabetes. The extensive study of 5,715 cases with a single neurodegenerative disease in the National Alzheimer’s Coordinating Centre USA database concluded that “in the absence of any specific disease-modifying treatments for Alzheimer’s disease in the near future, we urge, based on the high prevalence on cerebrovascular disease described in our data here, that aggressive management of vascular risk factors and encouragement of healthy life styles in mid-life may have benefit for Alzheimer’s disease or α-synucleinopathies individuals at increased risk to become clinically symptomatic, and probably to those with other causes of cognitive impairment. Indeed, even those who already manifest the clinical features of Alzheimer’s disease or α-synucleinopathy may benefit from effective therapies that mitigate vascular risk factors and cerebrovascular disease” (6).
Hypertension
Mid-life, but not late-life, hypertension is associated with an increased risk of AD and dementia with a calculated OR of 1.61(1.16–2.24) (28). A cohort of a random, population-based sample of 1449 individuals in Sweden was followed for an average of 21 years. Those with a raised systolic pressure in midlife (BP>160mm Hg) had a significantly higher risk of AD in later life OD 2.3 (1.0–5.5), after adjusting for age, body mass index, education, vascular effects, smoking and alcohol consumption (29). A quantitative meta-analysis of 14 studies of subjects without cognitive impairment or dementia, 32,658 with and 36,905 without hypertensive medication found no significant difference in the incidence of AD between the two groups but that those who had received anti-hypertensive medication has a significantly lower incidence of vascular dementia RR 0.67 (0.52–0.87) and of all-cause dementia RR 0.87 (0.7–-0.96) (30)
Hyperlipidaemia
A systematic review of 18 prospective studies found a significant association between high mid-life total cholesterol (TC) and an increased risk of AD and all-cause dementia but there was only weak evidence of an association between TC and cognitive decline (31).
Diabetes
A number of systematic reviews and meta-analyses have reported an increased risk of impaired cognition or dementia in association with Type-II diabetes (11). A meta-analysis of prospective 28 observational studies found that the pooled relative risk of developing AD was 1.56 (1.41–1.73) of VaD was 2.27 (1.96–2.66) and all-cause dementia was 1.73 (1.65-1.82) (32). Diabetes increased the risk of conversion of mild cognitive impairment to dementia and Mediterranean diet decreased the risk (33).
Obesity
In prospective studies and meta-analyses mid-life obesity has been found to be associated with a significant increase of all-cause dementia with a pooled estimate of RR of 1.60 (1.34–1.92) (17).
In addition to the specific effect of obesity on ARCID, obesity is associated with an increased incidence of hypertension, diabetes and cardiovascular disease.
Diet
A Mediterranean diet – high intake of vegetables, fruits, nuts and olive oil, relatively low intake of dairy products and red meat, and a moderate intake of wine – has been claimed in several observational studies to slow cognitive decline and to lower the risk of AD (34). In a prospective study of a similar “MIND” diet, high adherence was reported to be associated with a reduced risk of AD (35).
Smoking
A review of 37 studies found that compared with never smokers, current smokers had an increased risk of AD (RR1.40 (1.13–1.73), VaD (RR 1.38 (1.15-1.66) and all cause dementia (RR1.30 (1.13-1.73) (36). The risk of all-cause dementia increased by 34% for every 20 cigarettes smoked per day but was not increased in former smokers. In a study of a cohort of 21,123 people, heavy smoking in mid-life was associated during two decades of follow-up with a more than 100% increase in AD, VaD and all–cause dementia (37).
Physical Inactivity
A review and meta-analysis of 16 prospective studies on the association between physical activity and dementia found that comparing highest v lowest activity groups the combined RR for AD was 0.55 (0.36–0.84) and for all-cause dementia was 0.72 (0.60–0.80) (38). These values have been reversed to reflect the risks with inactivity as 1.82 (1.19–2.78) for AD and 1.39 (1.6–1.67) for all cause dementia (12). A review and meta-analysis of physical activity in 21 prospective cohorts comparing higher with lower levels of activity the RR on cognitive decline was 0.65 (0.55–0.76) and on dementia was 0.86 (0.76–0.97) (39). A Cochrane analysis in 2015 found that healthy, sedentary elders who begin exercise have a significant improvement in cognitive function, particularly mental processing speed (40).
Prevention of ageing-related cognitive impaiment and dementia: Combined measures
In the absence of disease-modifying treatments, measures to prevent or postpone the onset of ARCID should include measures to prevent cerebrovascular disease and improve physical health and to ensure an optimum psycho-social environment. In view of the long preclinical phase of both CVD and AD these measures need to be actively instituted as early in life as possible and not later than mid-life. Many studies of individual risk factors have been published but there have to date been virtually no long-term, randomized, controlled studies of combined measures to prevent or postpone ARCID. A recent Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) reported the results of a double-blind, randomized, controlled trial of 2,654 individuals aged 60-77 years assigned in a 1:1 ratio to multidomain intervention (cognitive training, diet, exercise and vascular risk monitoring) or a control group (general health advice) (41). The primary outcome was a change in cognition in a neuro-psychological test battery score (NTB). The difference in NTB between the two groups after 2 years was statistically significant (p=0.03). There was also a significant difference in the secondary outcomes of executive functioning (p=0.04) and processing speed (0.04) but not in memory (p=0.36). It was concluded that a multidomain intervention can improve cognitive function in at-risk elderly people.
Conclusion
Nine population-based studies of dementia incidence and prevalence have reported a declining prevalence and age-specific incidence of dementia in England, Sweden, The Netherlands and the USA (42). The decreases have been attributed to rising levels of education, better prevention and treatment of cardiovascular disease and healthier life-style including exercise. It is uncertain whether these favourable trends will continue in the face of rising levels of obesity and diabetes in these populations, and whether they will manifest in low income countries. Although the age-specific incidence of dementia may be decreasing in some countries, the population of the world, the number living to advanced old age and the number with dementia world-wide is increasing. The incidence of dementia rises rapidly over the age of 75 and it has been estimated that the total number of people with dementia will triple from 2015 to 2050. The best hope for reducing the incidence and prevalence of ageing-related dementia currently lies in primary prevention, and in particular better education, continued mental and physical exercise and strict control of vascular risk factors. The evidence is now more than sufficient evidence to urge the immediate implementation of both personal health and life-style measures and public health initiatives to prevent or delay the onset of ARCID and to decrease the burden world-wide.
Acknowledgements: I wish to acknowledge the authors M. Valenzuela, M Esler, K Ritchie and H Brodaty (8) and the publishers Translational Psychiatry ©Macmillan Publishers Limited for permission to publish Fig 1.
Conflict of interest: There are no conflicting interests. I have received no funds or writing assistance in preparation of the paper.
References
1. A. Alzheimer A. Uber eine eigenartige Erkankung der Hinrninde. Allegmine Zeitschrift fϋr Psychiatrie und Psychisch-GEritliche Medizin. 1907;64:146-8.
2. E.B. Larson, K. Kaffe,, K.M. Langa, New insights into the Dementia epidemic. N Engl J Med 2013;369:2275-7
3. S. Negash, D.A. Bennett, R.S. Wilson, J.A. Schneider, S.E. Arnold, Cognition and neuropathology in aging: multidimensional perspective from the Rush Religious Orders Study and Rush Memory Aging Project. Curr Alzheimer Res 2011;8:336-40.
4. S.B. Wharton, C. Brayne, G.M. Savva et al. Epidemiological neuropathology: the MRC Cognitive Function and Aging experience. J Alzheimers Dis 2011;25:359-72.
5. G.G. Kovacs, I. Milenkovic, A. Wohrer et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126:365-84.
6. J.B. Toledo, S.E. Arnold, K. Raibie, Contribution of cerebrovascular disease in an autopsy confirmed neurodegenerative disease case in the National Alzheimer’s Coordinating Centre. Brain 2013;136:2697-706
7. D.A. Davey. Alzheimer’s Disease, Cerebrovascular Disease and Dementia: a Potentially Preventable and Modifiable Syndrome, J Alzheimer’s Dis Parkinsonism 2015;5:1-5.
8. M. Valenzuela, M. Esler, K. Ritchie, H. Brodaty, Antihypertensives for combating dementia? A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry 2012 2,e107 doi: 10.1038/tp.2012.28.
9. D.A. Bennett, S.E. Arnold, M. J. Valenzuela, C. Brayne, J. A. Schneider, Cognitive and social life-style: links with neuropathology in late life. Acta Neuropathol 2014 127:137-50.
10. M.J. Valenzuela, P. Sachdev, Brain Reserve and dementia: a systematic review. Psychological Medicine, null441-4545 doi10.1017/S0033291705006264.
11. W. Xu, L. Tan, H-F. Wang H-F et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015 doi:10.1136/jnnp-2015-310548.
12. D. Barnes D, K. Yaffe. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10:819-28.
13. S. Norton, F.E. Matthews, D.E. Barnes, K. Yaffe, C. Brayne. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788-94.
14. W.A. Kukull, R.Higdon, J.D. Bowen et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 2002;59:1737-46.
15. K. Blennow, M.J. de Leon, H. Zetterberg, Alzheimer’s disease. Lancet 2006;368:387-403.
16. F. Caamano-Isorna, M. Corral. A. Monyes-Matrinez, B. Takkouche, Education and dementia: a meta-analytic study. Neuroepidemiology 2006;26:226-32.
17. M.A. Beydoun, H.A. Beydoun, A.A. Gamaldo, A. Teel, A.B. Zondeman, Y. Wang. Epidemiological studies of modifiable factors associated with cognition and dementia: systematic review and meta- analysis. BMC Public Health 2014;14:643-76.
18. R.S. Wilson, P.A. Scherr, J.A. Schneider, Y. Tang, D.A. Bennett. Relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology 2007;69:1911-20.
19. M. Martin, L. Clare, A.M. Altgassen, M.H. Cameron, F. Zehnder. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane database Syst Rev 2011(1):CD006220.
20. R.S. Wilson, K.R. Krueger, S.E. Arnold et al. Loneliness and risk of Alzheimer’s disease. Arch Gen Psychiatry 2007;64:234-40.
21. L. Fratiglioni. S. Paillard-Borg, B. Winblad, An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 2004;3:343-53.
22. P.A. Boyle, A.S. Buchman, L.L. Barnes, D.A. Bennett, Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry. 2010;67:304–310.
23. R.S. Wilson, J.A. Schneider, S.E. Arnold, J.L. Bienas, D.A. Bennett, Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry 2007;64:1204-12.
24. M. Valenzuela, C. Brayne, P. Sachdev, G. Wilcock, F. Matthews, Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol 2010;173:1004-12.
25. R.L. Ownby, E. Crocco, A. Acevedo, V. John, D. Loewenstein. Depression and risk for Alzheimer disease: systematic review, meta- analysis, and metaregression analysis. Arch Gen Psychiatry 2006;63:530-8.
26. S. Shively, A.L. Scher, D.P. Perl, R. Dias-Arrastia, Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 2012;69:1245-51.
27. D.E. Barnes, A. Kaup, K.A. Kirby, A.L. Byers, R. Diaz-Arrastia, K. Yaffe, Traumatic brain injury and risk of dementia in older veterans. Neurology 2014;83:312-9.
28. M. Baumgart, H.M. Snyder, M.C. Carrillo, S. Fazio, H. Kim, H. Johns, Summary of the evidence on modifiable factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia 2015;11:718-726.
29. M. Kivipleto, E.L. Helkala, M.P. Laakso et al. Midlife vascular risk factors and Alzheimer’s disease in later life: Longititudinal , population based study. BMJ 2011;322:1447-51.
30. H. Chang-Quan, W. Hui, W. Chao-Min et al. The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta-analysis of longitudinal studies. Int J Clin Pract 2011 65:1295 -305.
31. K.J. Anstey, D.M. Lipnicki, F. Low F, Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta- analysis. Am J Geriatr Psychiatry 2008;16:343-354.
32. K. Guadala, D. Bansal, F. Schifano, A. Bhansal. Diabetes Mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig 2013;4:3640-50.
33. C. Cooper, A. Sommerland, C.G. Lyketsos, G. Livingston. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 2015;172:323-34.
34. I. Lourida, M. Soni, J. Thompson-Coon et al. Mediterranean diet, cognitive function and dementia: a systematic review. Epidemiology 2013. 24:479-89.
35. M.C. Morris, C.C. Tagney, Y. Wang, T.M. Sacks, D.A. Bennett, N.T. Aggarwal, MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015 doi;10.1016/J.ALZ.2014.11.009.
36. G. Zhong, Y. Wang, Y. Zhang, J.J. Guo, Y. Zhao. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect of modifiers, PLoS One 2015.10;e118333. Doi10.1371/journal.pone0118333.
37. M. Rusanen, M. Kivipelto, C.P. Quesenberry Jr, J. Zhou, R.A. Whitmen, Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Int Med 2011. 171;333-9.
38. M. Hamer, Y. Chida, Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3-11.
39. S.J. Blondell, R. Hammersley-Mather, J.L. Veerman. Does physical activity prevent cognitive decline and dementia? A systematic review and meta- analysis of longitudinal studies. BMC Public Health 2014;14:510.
40. M. Angevaren, G. Aufdemkampe, H.J. Verhaar, A. Aleman, L. Vanhees. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane database of systematic reviews 2008 Jul 16;(3):CD005381. doi:1002/14651858.CD005391.pub2. Review. Update in Cochrane database of systematic reviews 2015 .4.CD00538
41. T. Ngandu, J. Lehtisalo, A. Solomons et al. A 2 year multidomain intervention of diet, exercise, cognitive training and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): randomized controlled trial Lancet 2015;385:2255-63.
42. K.M. Langa. Is the risk of Alzheimer’s disease and dementia declining Alzheimers Res Ther 2015:7;34.