V.M. Pak1, S. Newton2
1. PhD, MS, MTR; 2. MPH. Emory University, 1520 Clifton Road, Room 243, Atlanta, GA, USA
Corresponding Author: Victoria M. Pak, Emory University, 1520 Clifton Road, Room 243, Atlanta, GA 30326, USA, victoria.m.pak@emory.edu
J Aging Res Clin Practice 2018;7:91-99
Published online May 30, 2018, http://dx.doi.org/10.14283/jarcp.2018.17
Abstract
Abstract: Existing studies identify a possible link between choline, sleep disturbance, and Alzheimer’s disease, however further exploration is needed to determine the nature of this association. As the precursor to the neurotransmitter acetylcholine, choline plays an integral role in several neuronal processes, including some responsible for memory and learning. Decreased cholinergic neuronal activity is associated with brain abnormalities consistent with Alzheimer’s disease, an aging disease that disproportionately affects the elderly, and is believed to contribute to the cognitive decline experienced by Alzheimer’s disease patients, however the mechanism for this is not well understood. In this narrative review, we explore the associations between sleep disturbances, choline and the cholinergic pathway, and Alzheimer’s disease. Current research shows that the connection between sleep disturbances, choline, and Alzheimer’s disease is worth exploring in greater depth. In this review, we demonstrate there is a need for further studies to understand the mechanism through which inadequate sleep may impair the cholinergic pathway in order to guide targeted treatments for Alzheimer’s disease.
Key words: Alzheimer’s sleep disturbance choline cognitive decline sleep apnea.
Introduction
Alzheimer’s disease (AD) disproportionately affects the aged population, as approximately 30% of individuals over the age of 80 suffer from dementia, with the most common form of dementia being Alzheimer’s (1). Determining the risk factors for AD will help to reduce the prevalence of this disease, thereby improving the quality of life of the aged population. While cholinesterase inhibitors such as rivastigmine are commonly prescribed drugs for treatment of AD, adverse effects of these drugs (typically nausea, vomiting, weight loss, and dizziness) raise questions as to whether the clinical effects are economically worthwhile (2, 3). Current gene expression and metabolomic studies show that there is a relationship between choline, sleep, and brain development, thus more human studies are needed studying choline and the impact of sleep deprivation/sleep disturbance on cognitive decline. By pursuing –omic studies, these results will guide novel targeted Alzheimer’s disease therapies, resulting in better treatments for AD patients. This narrative review compiles the existing literature on Alzheimer’s disease, choline, and sleep disturbances in order to provide an update on the current understanding of the mechanism through which inadequate sleep may impair the cholinergic pathway, thereby potentially leading to cognitive deficits such as AD. We expect that the present review will inspire further –omic research and the impact of sleep disturbances on Alzheimer’s progression, in order to guide more targeted treatments of Alzheimer’s disease.
Link between choline and sleep disturbance
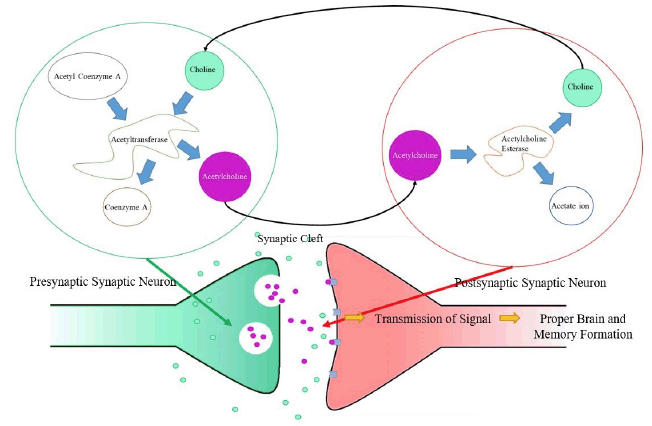
Several links have been established between sleep disturbances and choline. Sleep disturbance is any event that disrupts the sleep cycle, including insomnia, sleep disordered breathing, and sleep-related movement disorder (4), and is associated with excessive daytime sleepiness (5, 6). We have previously shown that low levels of choline are also linked to sleepiness symptoms (7) in subjects with suspected sleep apnea. Whether low choline is a result of disturbed sleep and subsequent sleepiness or subjects who exhibit sleepiness have underlying mechanisms that impact choline metabolism or endogenous production remains to be determined. Sleep disturbance has been shown to diminish choline plasmalogen levels in humans (8). A study that exposed rats to intermittent hypoxia during sleep demonstrated reduced choline acetyltransferase (ChAT) immunoreactivity in treated rats (9). This suggests that intermittent hypoxia during sleep was associated with a decrease in ChAT activity due to choline scarcity, the consequence of this being lowered production of acetylcholine (see pathway in Figure 1). As treated animals exhibited weakened spatial working memory, these findings also corroborate previous evidence of the association between lower ChAT activity and impaired memory function (9). Row et al. attributed this weakened memory to potentially diminished hippocampal and frontal cortical functioning as a result of lower ChAT activity.
Choline can be taken in through diet or produced by the body (through the breakdown of acetylcholine by acetylcholine esterase). Choline acetyltransferase catalyzes the reaction between choline (from the synaptic cleft) and acetyl coenzyme A (from the mitochondrial matrix) to form acetylcholine and coenzyme A as a byproduct (which is then used to produce more acetyl coenzyme A). Acetylcholine is then transferred from a presynaptic neuron to a post-synaptic neuron, allowing for a signal to be transmitted. In order to end transmission of a signal, acetylcholine esterase catalyzes the hydrolysis of acetylcholine before it reaches the post-synaptic neuron, forming choline and an acetate ion as a byproduct. The choline produced is then once again taken by acetyltransferase to produce acetylcholine, restarting the cycle, which allows for proper cognition and memory formation.
Evidence from several studies indicates that the link between sleep disturbance and decreased cholinergic neural function could be partially attributable to oxidative stress. Inadequate sleep leads to higher levels of oxidative stress-related gene expression in human blood (10). Oxidative stress has been shown to decrease ChAT activity and degrade cholinergic neurons in mice and in culture (11, 12). As such, oxidative stress is a likely mechanism through which insufficient sleep reduces choline levels and ChAT activity which remains to be studied.
Link between choline and sleep architecture
Previous research suggests that the deterioration of cholinergic neurons, indicative of Alzheimer’s disease (13), leads to lower delta generation (measured by electroencephalogram) during non-Rapid eye movement (REM) sleep (14). The cholinergic hypothesis of dementia attributes the learning and memory deficits of dementia in the aged and in AD to a decline of cholinergic systems of the basal forebrain (1). Basal forebrain cholinergic neurons are involved in cortical activation during waking hours and REM sleep by stimulating cortical activation with gamma and theta activity through the process of increasing discharge and firing in rhythmic bursts in response to transmitters of the activating systems (15). Thus, the cholinergic basal forebrain may play a central role in the regulation of the sleep-wake cycle via this dual mechanism through tonic firing (~7 Hz) during active wake and burst firing (~14 Hz) during REM (16, 17). In order to investigate the effects of acute cholinergic activation on the dynamics of sleep-to-wake transitions, Irmak and de Lecea, 2014, performed optogenetics, a technique to control or monitor neural activity with light, and electroencephalogram (EEG) on heterozygous Chat-IRES-cre 2-5 month old male mice to analyze transitions from non-REM sleep to REM sleep (18). Optogenetic activation of basal forebrain cholinergic neurons during non-REM sleep sufficiently facilitated transitions to wakefulness and arousal in their study, thereby demonstrating the role of the basal forebrain cholinergic system in inducing these states (18).
Association between sleep apnea and Alzheimer’s disease
A 2016 meta-analysis of five cross-sectional studies on the association between obstructive sleep apnea (OSA) and Alzheimer’s disease determined that AD patients are five times more likely to have OSA than cognitively normal individuals of the same age, and that about half of AD patients have at some point after their initial diagnosis of AD experienced obstructive sleep apnea [19].The studies determined OSA by either polysomnography, 24-chanel polygraph or respiratory inductive plethysmography (19). In a separate sleep study, obstructive sleep apnea (measured by polysomnography for three consecutive nights) was present in almost half of the 21 demented patients with probable AD compared to 23 healthy controls (20). Cardiovascular issues and changes in sleep quality associated with OSA may contribute to further cognitive decline and aggravate the progression and severity of AD (19, 21). A proposed mechanism of this association between obstructive sleep apnea and Alzheimer’s disease is that sleep fragmentation and a decrease in slow-wave sleep and REM related to OSA, and the maladaptive effects of intermittent hypoxia including neuroinflammation, neurotransmitter changes, cerebral amyloidogenesis and tau phosphorylation, lead to mild cognitive impairment, which in turn aggravates the progression of AD (22).
Link between choline and Alzheimer’s disease
Lowered ChAT and acetylcholinesterase (AChE) activity is the most common neurotransmitter deficit found in AD patients, compared to age-matched controls (23). Patients with severe Alzheimer’s disease exhibit 85-90% lower levels of ChAT and AChE, compared to the normal level, while patients with moderate AD display 35-50% lower ChAT levels than normal (24, 25). Additionally, it has been shown that decreasing levels of ACh synthesis (obtained via diagnostic craniotomy) are significantly correlated with cognitive decline in Alzheimer’s disease patients (25). A recent study examined lipid concentrations in postmortem prefrontal cortexes donated by Harvard Brain Tissue Resource Center of 10 AD patients and 9 non-AD controls. Investigators found a significant 73% lower level of plasmalogen choline in patients with AD, while other phospholipid levels remained unchanged, meaning lower choline levels are especially characteristic of AD (26). In another study, a mouse model of Alzheimer’s Disease (AD) showed that Tg2576 genetically engineered AD mice with age-dependent ß deposition in their brains displayed sleep abnormalities akin to those displayed in Alzheimer’s disease patients, compared to control mice, and hypothesized that this could be due to cholinergic deficiencies in AD mice (27). The mouse models of AD exhibited circadian periods greater than 24 hours and higher frequency oscillations in the sleep EEG than that of wild type controls. In addition, the increase in electroencephalographic delta (1–4 Hz) power that occurs during non-REM sleep after sleep deprivation was blunted in AD mice models compared to controls (27).
In addition, several studies have found correlations between genetic variation in the ChAT gene and Alzheimer’s disease. By utilizing the stochastic search variable selection (SSVS) approach to analyze association with multiple candidate genes simultaneously, Kim et al. (2004) found that a G +4 A polymorphism in the ChAT gene, in concert with Apolipoprotein E ε4, was associated with 3.25 higher odds of AD in a study of 246 AD patients and 561 controls (28). The investigators also found that the average ages-at-onset of AD were lower in those carrying the ChAT AA genotype than those carrying the ChAT AG or ChAT GG, regardless to the occurrence of the APOE ε4 (28). In addition, Ozturk et al. (2006) examined three SNPs in the ChAT gene in association with AD in 1001 white late-onset Alzheimer’s disease (LOAD) patients compared to 708 white controls, and found an increased risk of AD with exon 5 and intron 9 SNPs (29). Among non-APOE*4 carriers, intron 9, or a variant associated with intron 9 in the same haplotype, was also associated with AD risk in an APOE-dependent fashion (29). However, this finding is preliminary and should be confirmed in future studies with large samples. The investigators also found suggestive associations of all 3 SNPs (exon S, exon 5, intron 9) with cognitive function as measured by Mini Mental State Examination score (29).
As acetylcholine is known to play a complex and significant role in memory formation and coordination, lowered activity within the cholinergic pathway has been associated with memory impairment, as in AD (30, 31). Hasselmo proposes that during active waking (defined as a period characterized by large amplitude oscillations in the 3–10 Hz range in EEG recordings), cholinergic suppression of excitatory feedback reduces the influence of acetycholine on memory formation to a level that allows normal retrieval, but prevents distortion of the incoming sensory information being encoded, while during quiet waking and slow-wave sleep, lower levels of acetylcholine allow these excitatory feedback connections to have a stronger influence (30). For example, in a study of retired breeder adult mice, those with choline deficient diets (<1mg of choline per gram of chow) exhibited memory loss, while those with choline-rich diets (12-15mg of choline per gram of chow) showed less memory loss (32). In humans, a randomized, double blind, placebo-controlled study demonstrated that verbal memory in older adults improves with 1000 mg/d dietary citicoline supplementation (a metabolic intermediate that completely dissociates to choline and cytidine upon entering the body) (33). In another double-blind study, patients with Alzheimer’s disease given 20-25 g/day of the choline precursor purified soya lecithin (containing 90% phosphatidyl plus lysophosphatidyl choline) for six months exhibited moderate improvements on multiple tests measuring orientation, verbal learning, memory, and self-care (34). However these results were seen only among a small sub-group of older patients that were relatively poor compliers (<75% of the prescribed dose of phosphatidylcholine) and had intermediate levels of plasma choline (34). Ultimately, the cholinergic pathway’s important role in memory function suggests that low choline levels or ChAT/AChE activity may be contributing to memory impairment in AD. However, these studies have focused mainly on dietary choline and its associations with memory, and have not addressed the association between sleep, choline, and memory.
Gene expression and choline in Alzheimer’s disease
Gene expression has been conducted on AD patients to assess ChAT levels using in situ hybridization in the caudate nucleus, putamen and ventral striatum (13). Results showed decreased ChAT mRNA expression within cholinergic neurons of the ventral striatum, especially in those most vulnerable to the neurodegenerative process (13). The down-regulation of ChAT mRNA may amplify the loss of cholinergic neurons within the ventral striatum, whose numbers have been shown to already be reduced within AD patients post-mortem via immunohistochemistry (35, 36). Another gene expression study examined ChAT activity and expression in the basal forebrain during periods of behavioral activity and throughout sleep-wake states. A high level of ChAT mRNA expression during REM sleep was shown, and an intermediate level was found during slow-wave sleep and a low level during wakefulness (37). The data also showed an inverse relationship with ChAT protein activity, as higher activity was associated with wakefulness and behavioral activity, and reduced protein activity was observed during sleep (37). A recent study examined the role of cholinergic basal forebrain neurons in the transcriptomic response to sleep deprivation. The study assessed mRNA expression in cholinergic cells using microarrays in sleeping and sleep-deprived mice to characterize cholinergic cell signatures, finding the expression of clock genes (Arntl2, Per1, Per2, Dbp, Nr1d1) as well as mGluR3 in these cells (38). In choline-deficient mouse embryos, expression of multiple miRNAs is changed in neural progenitor cells (39). A recent study examined miRNA-mediated regulation of mRNA transcripts involved in the synthesis, vesicle packaging, and destruction of ACh (40). Data indicates massive overlap of these miRNAs with those regulating ACh degradation, indicating a new surveillance mechanism of the cholinergic signaling pathway by way of the regulation of acetylcholinesterase degradation by miRNAs, which may be of value for both basic and translational aspects of neuroinflammation-related disorders (40). Examining miRNA levels in the blood may be ideal, due to it being more easily accessible and less invasive in patients. Gene expression in the blood of rats was shown to co-express about 56% of genes in brain tissue (41). Exploring miRNA in ACh will be important to guide targeted treatments for sleep disturbances and AD. A previous review has described the role of the cholinergic system within the reticular acting system (RAS), which simultaneously induces and modulates REM sleep by way of ascending projections to the intralaminar thalamus (ILT) to modulate thalamocortical systems throughout sleep-wake states, and descending projections to the pontomedullary reticular formation (42). It is clear that there is a relationship between choline, sleep disturbances, and brain development, thus human studies are needed on gene expression, choline and associated metabolites and the impact of sleep deprivation/sleep disturbance such as sleep apnea on Alzheimer’s progression.
Link between choline and cognitive effects
Choline is known to play a significant role in cognitive processes. As the precursor to the neurotransmitter acetylcholine, choline plays an integral role in several neuronal processes, including some responsible for memory and learning (43). Decreased cholinergic neuronal activity is associated with brain abnormalities consistent with Alzheimer’s disease (AD) and is believed to contribute to the cognitive decline experienced by AD patients (1, 43, 44). The high-affinity choline uptake transporter (CHT) brings in choline from the extracellular space to presynaptic terminals, thereby enabling normal acetylcholine synthesis and cholinergic transmission (45). Thus, reductions in CHT capacity have been associated with decreased ability to perform tasks that require attentional processes (45). For example, animals’ performance on activities like the 5-choice serial reaction time task (assesses divided and sustained attention) has been associated with increases in cortical cholinergic inputs and cortical acetylcholine efflux (45). In a sustained attention task that required rats to detect the presence and absence of signals that occur unpredictably, the release of cortical Acetylcholine (ACh) in the frontoparietal cortex was higher than in control tasks that did not require attentional processes, highlighting the important role of choline in attentional processing (46). In another experiment, infusions of the cholinotoxin 192 IgG-saporin on 12 male BNNia/F344 four-month old rats induced loss of cortical cholinergic inputs, which in turn resulted in the rats’ impaired performance in a sustained attention test, while performance on non-attention related control activities was unaffected (47). The sustained attention test was designed to measure vigilance by requiring subjects to respond to the presentation of visual signals. Choline has also been shown to hold critical developmental importance for memory (48, 49). Sprague-Dawley pregnant rats’ dietary choline intake has a significant impact on hippocampal brain development, to such an extent that rats whose mothers had extra dietary choline can be identified even when the rats are elderly, based on their memory function (50). Conversely, when rodent mothers are choline-deficient during pregnancy, their offspring have persistent memory and cognitive impairments (49). A recent study showed that postnatal choline supplementation of daily choline injection (100 mg/kg) in ethanol-exposed and control rats improved working memory function, especially when combined with working memory training (51). In rat models of traumatic brain injury (TBI), a choline-supplemented diet with concentration of 2% choline was shown to improve spatial memory and normalize some TBI-induced effects in nicotinic cholinergic receptor (nAChR) expression (52). In a study of 1391 patients free of neurological conditions, dietary choline intake (measured by a food frequency questionnaire), was shown to be associated with better verbal and visual memory (53). Thus, choline appears to play a critical role in attentional processes (and by extension, learning processes, which are closely related), as well as memory (45).
In a 2010 randomized control trial with 76 asthma patients, choline supplementation (1500 mg, twice daily) for six months was shown to attenuate immune inflammation and suppress oxidative stress (54). Therefore, choline may decrease inflammation/oxidative stress and its subsequent cognitive effects. While the mechanism underlying this process remains to be determined, a potential explanation may be the protective effect of the nAChR agonist on the development of airway inflammation through the cholinergic anti-inflammatory pathway (55, 56), and choline may be mediated through the activation of alpha-7-nicotinic receptor. In a study of post-operative mice, administration of choline prior to surgery markedly reduced the proinflammatory cytokine response to surgery and ameliorated memory impairment (57). A recent study observed the benefits of a daily dose of 52mg of choline for five weeks in healthy adult rats, finding that rats receiving choline showed improved cognitive and locomotor performance and a reduction in oxidative stress (58). The investigators state that the reduction in oxidative stress is due to choline decreasing the levels of lipid peroxidation, stimulating the activities of antioxidant enzymes and increasing the antioxidant stores of GSH and proteins, thereby reducing the production of reactive oxygen species (ROS) (58). The contribution of cholinergic dysfunction in the development of cognitive impairments, such as AD, could become an important biomarker in identifying high-risk patients for these impairments and therefore needs to be further explored.
Current human epidemiological studies on choline, sleep disturbances, and Alzheimer’s disease
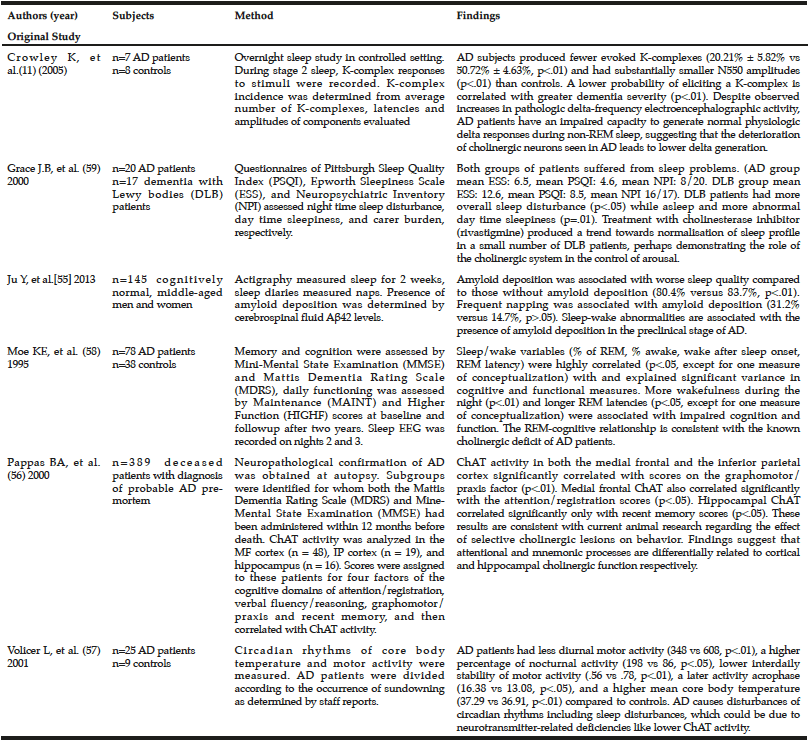
Recent studies have demonstrated various potential links between sleep disturbances, choline, and AD in humans (Table 1). In one study of seven patients with AD and eight controls in a research sleep laboratory, researchers discerned the difference between sleep-related delta and pathological delta activity, suggesting that the deterioration of cholinergic neurons seen in AD leads to lower physiologic delta generation (a high-amplitude brain wave associated with slow-wave sleep) compared to age-matched controls (14). However, the small sample size of this study raises concerns about whether it is representative of the population with AD. Patients with pre-clinical AD (as measured by amyloid deposition) have also been shown to have lower quality sleep), as one cross-sectional study with 145 participants demonstrated (59). Sleep quality was measured objectively over two weeks with an actigraph worn on the patient’s wrist. This study postulated that poor sleep might also play a role in amyloid deposition because chronic sleep deficiency increases neuronal activity, which leads to an excess of soluble Aß that could eventually become plaques (59). Though this study had a large sample size, it did not examine other markers of pre-clinical AD, including choline levels or ChAT/AChE activity. The study also did not assess for any other sleep-related variables, other than sleep quality and sleep quantity, for association with amyloid deposition.
One study of post-mortem ChAT activity found that lower ChAT activity in AD patients was associated with significantly lower previous scores on the Mattis Dementia Rating Scale and the Mini-Mental State Examination, especially in the graphomotor/praxis, attention/registration, and recent memory scores (60). However, this study did not address how sleep may play into these choline and memory impairment dynamics. A study of circadian rhythms of motor activity and core body temperature (measured with a temperature-sensitive thermistor probe and recorded every 6 minutes for 72 hours) in 25 people with AD and 9 healthy people revealed that AD patients displayed less daytime motor activity, more nighttime activity, and less motor activity stability between days (61). The authors concluded that AD causes disturbances of circadian rhythms (61). In addition, AD patients’ core temperature was higher on average, and their temperature peaked later, compared to healthy subjects (61). The researchers proposed that these changes, including sleep disturbances, could be due to neurotransmitter-related deficiencies like lower ChAT activity. However, the study did not look for evidence on the mechanism through which lower ChAT activity could lead to disrupted circadian rhythms. A prior study examined memory and cognition (as assessed by Mini-Mental State Examination and Dementia Rating Scale), and daily functioning, as they relate to sleep (measured by EEG) among 78 AD patients and 38 non-AD controls (62). The authors observed that more wakefulness during the night and longer REM latencies were associated with impaired cognition and function, which is consistent with the known cholinergic deficit of AD patients (62). A prior study measured the night time sleep disturbance (Pittsburgh Sleep Quality Index) and day time sleepiness (Epworth Sleepiness Scale) among 20 AD patients and 17 dementia Lewy bodies patients, and found a high level of sleep disturbance in both patient groups (63). Within a small group of dementia patients, rivastigmine (a cholinesterase inhibitor) reduced daytime sleepiness and nighttime sleep disturbance, and resulted in a higher MMSE score for cognitive functioning, suggesting that the cholinergic system plays a role in the control of arousal (63). However, in this study rivastigmine was not tested in AD patients. Additionally, cognitive function of AD patients was assessed only at baseline.
A review of the literature on the cholinergic pathway and circadian rhythms put forward the hypothesis that cholinergic neurotransmission in the SCN supports “time stamping,” a memory of a certain time of day, which in turn aids the development of time memory (64). Time stamping requires the engagement of muscarinic acetylcholine receptors (mAChRs) (64). Internalized mAChRs make cholinoceptive cells less sensitive to behavioral arousal accompanied by enhanced levels of ACh through the mechanism of lowering these cells’ sensitivity to ACh (64). These internalized mAChRs reduce or block further cholinergic input, protecting cholinoceptive cells and therefore the initiated circadian rhythm from disruption (64). Therefore, without time stamping, the authors postulate that circadian rhythms and memory formation could become disrupted, as in AD. Indeed, a 2008 review of literature on sleep, memory and AD suggests that memory processes are modulated by a circadian rhythm in central cholinergic transmission, with high ACh levels during wakefulness and reduced levels during slow-wave sleep (65). However this review is from 2008 and focuses on the treatment of AD with cholinesterase inhibitors, specifically galantamine. A more robust review of the literature over the past decade would aid in future research on AD, sleep, and choline.
Choline treatment for sleep disturbances
Plasma choline levels are directly affected by daily choline intake (66). If sleep and choline are interrelated as past literature has suggested (15-18), it is logical to pursue studies examining dietary choline and sleep disturbances. According to a study reporting data from the National Health and Nutrition Examination Survey (NHANES), in which 5,587 patients were administered questionnaires assessing their nutrient intake, sleep habits and physical activity by 24-hour recall during in-person interviews conducted in the home, lower dietary choline intake has been associated with a slightly higher likelihood of having 9+ hours sleep duration (RR = 0.45), when adjusting for other dietary habits (67). The results suggest that lower choline intake is associated with higher levels of sleepiness, or that lower choline intake is simply associated with more sleep, leading to less sleepiness. As the existing literature discusses a possible link between sleep disturbance and choline deficiency, dietary choline’s potential to treat sleepiness or improve sleep architecture has yet to be examined. Additionally, due to the use of questionnaire to assess subjects’ nutrition and sleep habits, sleep duration and dietary intake are self-reported and thus limited. The cross-sectional nature of the study also limits the ability to determine the direction of the association between sleep and dietary intake of choline extrapolated from questionnaire which is not reliable. The duration of sleep could impact choline intake, or vice-versa. Exploring treatment for sleepiness and sleep disturbances with choline is important due to the overlap of sleep disturbances/ memory associated with choline. Treatment for sleepiness and sleep disturbances will likely guide treatments for Alzheimer’s.
Choline treatment for Alzheimer’s disease
Choline has been shown to improve memory outcomes among AD patients, as in Spiers et al. (1996), a randomized controlled trial in which AD patients who ingested 2000mg/d of citicoline exhibited better immediate and delayed recall on logical memory compared to a placebo group (33). Four additional clinical trials of dietary citicholine for AD patients showed that treated patients attained better scores on cognitive evaluation scales, compared to control groups (68-71). With regard to choline alfoscerate, a double-blind randomized-controlled trial found that treatment of 400mg of choline alfoscerate three times a day resulted in significantly improved scores on the Mini-Mental State Examination, the Global Deterioration Scale, the Alzheimer’s Disease Assessment Scale (Behavioral and Total), and the Global Impression Scale after 90 days within patients with mild-moderate AD, while the placebo group scores remained largely the same or worsened (72). Mild-moderate AD was determined by the following: diagnosis of probable or possible AD according to the Diagnostic and Statistical Manual of Mental Disorders (73) and National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (74); Mini-Mental State Examination™ (75)(MMSE) score between 12 and 26; Modified Hachinski Score (76) (M-HIS) <4. Several other studies have found similarly positive outcomes with the use of choline alphoscerate supplementation for patients with AD (77). However, not all cholinergic precursors have proven effective treatments for AD—a review of clinical trials of cholinergic precursors has shown that supplementation of lecithin or phosphatidylserine resulted in better subjective memory, but did not ultimately improve outcomes including quality of life, cognitive function, and death, for AD patients (78). The reasons for the lack of effect of this precursor therapeutic strategy remain unclear. In spite of the numerous investigations, not all aspects of deposition and metabolism of choline and of its utilization for biosynthesis of ACh were clarified yet (77). Thus, it appears that different cholinergic precursors have different effects, and further studies are needed to explore these precursors to guide effective treatments for AD patients.
Future directions
Although many studies have addressed insufficient sleep’s detrimental effects on the cholinergic pathway, low choline/ChAT/AChE’s levels’ association with Alzheimer’s disease, and AD’s association with low sleep quality, few have addressed the relationship between sleep disturbances, the cholinergic pathway, and AD. Are cholinergic deficiencies a risk factor for AD, or a characteristic of the disease itself, or both? As AD is associated with subjectively and objectively measured (via PSQI and actigraph, respectively) poor sleep quality, is poor sleep quality associated with an increased risk of AD or is poor sleep quality a sign of pre-clinical AD, or both? In this instance poor sleep quality refers to sleep efficiency (total sleep time divided by time in bed, and wake time after sleep onset). Future studies are needed to explore whether dietary/supplemental choline intake improves sleep quality. While the aforementioned studies have addressed some of these questions, their complexity merits further exploration. Also, while mouse models have proven valuable in examining altered cholinergic systems and their effects on memory, human studies with larger samples are needed to effectively examine the links between sleep disturbance, choline, and Alzheimer’s disease.
In addition, the mechanism through which inadequate sleep may impair the cholinergic pathway needs further study—does sleep disturbance lower choline levels and ChAT/AChE activity through oxidative stress, inflammation, or another mechanism? It would be important to clarify the mechanism of which insufficient sleep degrades other neurotransmitters or parts of the brain that also play a role in memory and attentional processes. This narrative review compiles the existing literature, showing that the connection between sleep disturbances, choline, and AD is worth exploring in greater depth in order to guide novel therapies for Alzheimer’s disease patients.
Conflict of interest: The authors have no conflict of interests to report.
Ethical Standards: The authors complied with ethical guidelines in this review. This study did not use human subjects.
References
1. Muir JL. Acetylcholine, aging, and Alzheimer’s disease. Pharmacol Biochem Behav. 1997;56(4):687-696.
2. Tan CC, Yu JT, Wang HF, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615-631.
3. Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2015;9:CD001191.
4. Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2017.
5. Cote KA, Milner CE, Osip SL, Ray LB, Baxter KD. Waking quantitative electroencephalogram and auditory event-related potentials following experimentally induced sleep fragmentation. Sleep. 2003;26(6):687-694.
6. Slater G, Steier J. Excessive daytime sleepiness in sleep disorders. J Thorac Dis. 2012;4(6):608-616.
7. Pak VM, Dai, F., Keenan, B., Gooneratne N., Pack, A. Lower Plasma Choline Levels are Associated with Sleepiness Symptoms. Sleep Medicine. 2017:In Press.
8. Chua EC, Shui G, Cazenave-Gassiot A, Wenk MR, Gooley JJ. Changes in Plasma Lipids during Exposure to Total Sleep Deprivation. Sleep. 2015;38(11):1683-1691.
9. Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177(2):308-314.
10. Moller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):E1132-1141.
11. Flavin MP, Yang Y, Ho G. Hypoxic forebrain cholinergic neuron injury: role of glucose, excitatory amino acid receptors and nitric oxide. Neuroscience Letters. 1993;164(1–2):5-8.
12. McKinney M, Jacksonville MC. Brain cholinergic vulnerability: Relevance to behavior and disease. Biochemical Pharmacology. 2005;70(8):1115-1124.
13. Boissiere F, Faucheux B, Agid Y, Hirsch EC. Choline acetyltransferase mRNA expression in the striatal neurons of patients with Alzheimer’s disease. Neurosci Lett. 1997;225(3):169-172.
14. Crowley K, Sullivan E, Adalsteinsson E, Pfefferbaum A, Colrain I. Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep. 2005;28(7):865-870.
15. Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157-169.
16. Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8(11):4007-4026.
17. Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84(3):1627-1635.
18. Irmak SO, de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37(12):1941-1951.
19. Emamian F, Khazaie H, Tahmasian M, et al. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front Aging Neurosci. 2016;8:78.
20. Reynolds CF, 3rd, Kupfer DJ, Taska LS, et al. Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. J Clin Psychiatry. 1985;46(7):257-261.
21. Bliwise DL. Alzheimer’s disease, sleep apnea, and positive pressure therapy. Curr Treat Options Neurol. 2013;15(6):669-676.
22. Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3(5):404-414.
23. Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408-414.
24. Giacobini E. Cholinergic function and Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2003;18(S1):1099-1166.
25. Francis PT. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005;10(11 Suppl 18):6-9.
26. Igarashi M, Ma K, Gao F, Kim H-W, Rapoport SI, Rao JS. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. Journal of Alzheimer’s Disease. 2011;24(3):507-517.
27. Wisor JP, Edgar DM, Yesavage J, et al. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer’s disease: A role for cholinergic transmission. Neuroscience. 2005;131(2):375-385.
28. Kim K-W, Suh Y-J, Park W-Y, et al. Choline acetyltransferase G +4 A polymorphism confers a risk for Alzheimer’s disease in concert with Apolipoprotein E ε4. Neuroscience Letters. 2004;366(2):182-186.
29. Ozturk A, DeKosky ST, Kamboh MI. Genetic variation in the choline acetyltransferase (CHAT) gene may be associated with the risk of Alzheimer’s disease. Neurobiology of Aging. 2006;27(10):1440-1444.
30. Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends in Cognitive Sciences. 1999;3(9):351-359.
31. Micheau J, Marighetto A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behavioural Brain Research. 2011;221(2):424-429.
32. Bartus RT, Dean RL, Goas JA, Lippa AS. Age-related changes in passive avoidance retention: modulation with dietary choline. Science. 1980;209(4453):301-303.
33. Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Archives of neurology. 1996;53(5):441-448.
34. Little A, Levy R, Chuaqui-Kidd P, Hand D. A double-blind, placebo controlled trial of high-dose lecithin in Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. 1985;48(8):736-742.
35. Lehericy S, Hirsch EC, Cervera P, et al. Selective loss of cholinergic neurons in the ventral striatum of patients with Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(21):8580-8584.
36. Selden N, Geula C, Hersh L, Mesulam MM. Human striatum: chemoarchitecture of the caudate nucleus, putamen and ventral striatum in health and Alzheimer’s disease. Neuroscience. 1994;60(3):621-636.
37. Greco MA, McCarley RW, Shiromani PJ. Choline acetyltransferase expression during periods of behavioral activity and across natural sleep-wake states in the basal forebrain. Neuroscience. 1999;93(4):1369-1374.
38. Nikonova EV, Gilliland JD, Tanis KQ, et al. Transcriptional Profiling of Cholinergic Neurons From Basal Forebrain Identifies Changes in Expression of Genes Between Sleep and Wake. Sleep. 2017;40(6).
39. Trujillo I SN, Wang Y, Zeisel SH. DNA Methylation and microRNA Expression are Altered by Choline Deficiency During Mouse Brain Development. The FASEB Journal. 2016;30(1).
40. Nadorp B, Soreq H. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front Mol Neurosci. 2014;7:9.
41. Witt SH, Sommer WH, Hansson AC, Sticht C, Rietschel M, Witt CC. Comparison of gene expression profiles in the blood, hippocampus and prefrontal cortex of rats. In Silico Pharmacol. 2013;1:15.
42. Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31(5):673-690.
43. Davies P. Challenging the cholinergic hypothesis in Alzheimer disease. JAMA. 1999;281(15):1433-1434.
44. Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269-296.
45. Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6(1):48-56.
46. Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114(2):451-460.
47. McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behavioral neuroscience. 1996;110(2):247-265.
48. Zeisel SH. Choline: Needed for Normal Development of Memory. Journal of the American College of Nutrition. 2000;19(sup5):528S-531S.
49. Zeisel SH. The fetal origins of memory: The role of dietary choline in optimal brain development. The Journal of Pediatrics. 2006;149(5, Supplement):S131-S136.
50. Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12(3):349-357.
51. Waddell J, Mooney SM. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients. 2017;9(10).
52. Guseva MV, Hopkins DM, Scheff SW, Pauly JR. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
53. Poly C, Massaro JM, Seshadri S, et al. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am J Clin Nutr. 2011;94(6):1584-1591.
54. Mehta AK, Singh BP, Arora N, Gaur SN. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. 2010;215(7):527-534.
55. Gallowitsch-Puerta M, Tracey KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann N Y Acad Sci. 2005;1062:209-219.
56. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384-388.
57. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986-995.
58. Tabassum S, Haider S, Ahmad S, Madiha S, Parveen T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol Biochem Behav. 2017;159:90-99.
59. Ju Y, McLeland J, Toedebusch C, al e. Sleep quality and preclinical alzheimer disease. JAMA Neurology. 2013;70(5):587-593.
60. Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Choline acetyltransferase activity and cognitive domain scores of Alzheimer’s patients. Neurobiology of Aging. 2000;21(1):11-17.
61. Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and Circadian Rhythms in Alzheimer’s Disease. American Journal of Psychiatry. 2001;158(5):704-711.
62. Moe KE, Vitiello MV, Larsen LH, Prinz PN. Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res. 1995;4(1):15-20.
63. Grace JB, Walker MP, McKeith IG. A comparison of sleep profiles in patients with dementia with lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2000;15(11):1028-1033.
64. Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behavioural Brain Research. 2011;221(2):466-480.
65. Nieoullon A, Bentue-Ferrer D, Bordet R, Tsolaki M, Forstl H. Importance of circadian rhythmicity in the cholinergic treatment of Alzheimer’s disease: focus on galantamine*. Curr Med Res Opin. 2008;24(12):3357-3367.
66. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)1998.
67. Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71-80.
68. Caamano J, Gomez MJ, Franco A, Cacabelos R. Effects of CDP-choline on cognition and cerebral hemodynamics in patients with Alzheimer’s disease. Methods and findings in experimental and clinical pharmacology. 1994;16(3):211-218.
69. Franco-Maside A, Caamano J, Gomez MJ, Cacabelos R. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods and findings in experimental and clinical pharmacology. 1994;16(8):597-607.
70. Cacabelos R, Caamano J, Gomez MJ, Fernandez-Novoa L, Franco-Maside A, Alvarez XA. Therapeutic effects of CDP-choline in Alzheimer’s disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann N Y Acad Sci. 1996;777:399-403.
71. Alvarez XA, Mouzo R, Pichel V, et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods and findings in experimental and clinical pharmacology. 1999;21(9):633-644.
72. De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clinical Therapeutics. 2003;25(1):178-193.
73. American Academy of Family Physicians., American Psychiatric Association. Work Group on DSM-IV-PC. Diagnostic and statistical manual of mental disorders, fourth edition : primary care version. 1st ed. Washington, DC: American Psychiatric Association; 1995. xv, 223 p. p.
74. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
75. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
76. Loeb C, Gandolfo C. Diagnostic evaluation of degenerative and vascular dementia. Stroke. 1983;14(3):399-401.
77. Amenta F, Parnetti L, Gallai V, Wallin A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mechanisms of Ageing and Development. 2001;122(16):2025-2040.
78. Higgins JP, Flicker L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst Rev. 2000(4):CD001015.