K.M. Hayden1, A. Anderson2, A.P. Spira3,4,5, M.-P. St-Onge6, J. Ding7, M. Culkin1, D. Molina-Henry8,9,
A.H. Sanderlin7,10, D. Reboussin2, J. Bahnson2, M.A. Espeland2,7
1. Department of Social Sciences and Health Policy, Wake Forest University School of Medicine, Winston-Salem, NC, USA; 2. Department of Biostatistics and Data Science, Wake Forest University School of Medicine, Winston-Salem, NC, USA; 3. Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; 4. Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, MD, USA; 5. Johns Hopkins Center on Aging and Health, Baltimore, MD, USA; 6. Department of Medicine, Columbia University Irving Medical Center, New York, NY, USA; 7. Department of Internal Medicine, Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA; 8. Winston-Salem State University, Winston-Salem, NC, USA; 9. University of Southern California, Alzheimer’s Therapeutic Research Institute, San Diego, CA, USA; 10. Department of Biology, North Carolina Agricultural and Technical State University, Greensboro, NC, USA
Corresponding Author: Kathleen M. Hayden, Department of Social Sciences and Health Policy, Wake Forest University School of Medicine, 1 Medical Center Boulevard Winston-Salem, NC 27157, Tel: (336) 716-2918, E-mail: khayden@wakehealth.edu
J Aging Res & Lifestyle 2023;12:46-55
Published online June 26, 2023, http://dx.doi.org/10.14283/jarlife.2023.9
Abstract
BACKGROUND: Daytime sleepiness is common in older adults and may result from poor nighttime sleep due to sleep disordered breathing, fragmented sleep, or other sleep disorders. Daytime sleepiness may be associated with cognition in older adults.
OBJECTIVES: We investigated the association between self-reported daytime sleepiness and cognitive function in the Look AHEAD clinical trial.
DESIGN: Observational follow-up of a randomized clinical trial of an intensive lifestyle intervention.
SETTING: Clinic.
PARTICIPANTS: Participants (n=1,778) aged 45-76 years at baseline with type 2 diabetes and overweight or obesity.
INTERVENTIONS: Participants were randomized to an intensive lifestyle intervention for weight loss or a control condition of diabetes support and education.
MEASUREMENTS: Participants provided self-reported levels of daytime sleepiness at baseline and years 12-13. Cognitive function was assessed with a neurocognitive battery at years 12-13 and 18-20.
RESULTS: Participants who reported having frequent daytime sleepiness (often or always) performed significantly worse than others on the cognitive composite (-0.35; p-value=0.014) after controlling for covariates. When stratified by intervention arm, participants assigned to the intensive lifestyle intervention who reported often/always having daytime sleepiness performed worse on Digit Symbol Coding (-0.63; p-value=0.05) and Trail Making Part-B (-0.56; p-value=0.02) after controlling for covariates. Statistical interactions revealed associations between daytime sleepiness and the following covariates: race and ethnicity, APOE ε4 carrier status, baseline history of cardiovascular disease, and depression.
CONCLUSIONS: Daytime sleepiness over ~13 years predicted poorer cognitive performance in older individuals who, by virtue of having diabetes and overweight/obesity, are at high risk for sleep disorders and cognitive impairment.
Key words: Sleep disorders, diabetes mellitus, type 2, cognition disorders, aging, obesity, overweight.
Introduction
Daytime sleepiness is common in older adults (1, 2) and may signal health problems (3). Daytime sleepiness may result from sleep-disordered breathing, fragmented sleep or other sleep disorders (2), or from insufficient sleep, and it has been associated with comorbidities, medication use, and metabolic syndrome (4). The Cardiovascular Health Study showed associations between daytime sleepiness and incident cardiovascular disease (CVD) and mortality (3), and other studies have shown associations with cognitive decline (5, 6) and increased dementia risk (7, 8). Daytime sleepiness may signify disruption of circadian rhythms and may occur due to disorder in neural circuitry that may be affected in neurodegenerative disease including impairment in arousal systems (9). The locus coeruleus noradrenergic arousal system is associated with wakefulness and attention (10) and is affected early in the progression of neurodegeneration in Alzheimer’s disease (AD) (11). Self-reported excessive daytime sleepiness has been associated with later beta amyloid deposition, a pathological feature of AD, in the Baltimore Longitudinal Study of Aging (12).
Conditions associated with excessive daytime sleepiness and AD risk include obesity and diabetes (13). Among those with obesity and metabolic syndrome, heightened sympathetic activity can potentially cause fragmented sleep, leading to daytime sleepiness (14). Another factor, sleep-disordered breathing, could play a role, however daytime sleepiness among those with obesity or diabetes is not always associated with sleep-disordered breathing. Indeed, in a large study (n=16,583) of men and women, excessive daytime sleepiness was more strongly associated with greater body mass index (BMI), diabetes, and depression, than with sleep disordered breathing (13).
Previously in the Look AHEAD study, weight loss was shown to improve indices of sleep-disordered breathing and even remission of obstructive sleep apnea (OSA) (15), however daytime sleepiness was not studied. Look AHEAD provides the opportunity to evaluate the potential effects of a lifestyle intervention on daytime sleepiness and subsequent effects on cognitive function in a large, well-characterized cohort. Further, the large sample with cognitive assessments in Look AHEAD facilitates sub-analyses of gender differences and potential differences by APOE ε4 status, two important dementia risk factors, as well as subgroups for which we have found significant interactions in prior work (16-18) including age, baseline BMI, and baseline history of CVD. The objective of this analysis was to determine the degree to which self-reported daytime sleepiness was associated with the intervention and with cognition.
Methods
The study design, methods (19), and CONSORT diagram (20) for Look AHEAD have been published. Briefly, Look AHEAD was a randomized controlled clinical trial of (n=5,145) participants aged 45-74 with diabetes and overweight/obesity. The trial was designed to determine whether intentional weight loss is appropriate for older adults with diabetes and overweight/obesity, with primary end points of fatal and nonfatal cardiovascular events. Eligibility criteria required that participants have BMI >25 kg/m2 (>27
kg/m2 if on insulin), glycated hemoglobin (HbA1c) <11%, systolic/diastolic blood pressure <160/100 mmHg, and triglycerides <600 mg/dl. Participants were required to demonstrate over a two-week run-in period, the ability to record daily, their diet and physical activity. Each participant met with a behavioral psychologist or interventionist to confirm that intervention requirements were understood and that participants did not have any competing life stressors that would impair adherence to the protocol. Study data were collected by certified, trained staff who were masked to intervention assignments (19). Participants were randomly assigned with equal probability to either the intensive lifestyle intervention (ILI) or diabetes support and education (DSE) arm of the trial. Enrollment and initiation of intervention delivery occurred between 2001 and 2004. Interventions continued until 2011, at which time participants were invited to join a follow-up observational study to determine the longer-term effects of the intervention on outcomes. The average intervention duration for participants in this study was 9.8 years [8.4-11.1y]. Local Institutional Review Boards approved the protocols and all participants provided written informed consent.
The ILI was a multidomain intervention including dietary modification and increased physical activity with a goal of inducing an average of ≥7% weight loss at one year and maintenance of weight loss over the course of the study (21). Participants in the ILI arm were given a daily calorie goal of 1200-1800 kcal based on initial weight. The diet specified <30% total calories from fat (<10% saturated fat) and a minimum of 15% total calories from protein. The physical activity goal was similar in intensity to brisk walking for at least 175 minutes/week. Participants randomized to the DSE condition were invited, but not required, to attend three group sessions/year. Sessions focused on diet, physical activity, and social support (22). There were no specific instructions or goals for weight loss, physical activity, or dietary modification.
Daytime Sleepiness
At baseline and during extended post-intervention follow-up (12-13 years later), participants were asked about daytime sleepiness: “How often do you feel excessively(overly) sleepy during the day.” Responses included never(1 day/month or less), sometimes(2-4 days/month), often(5-15 days/month), and almost always(16-30 days/month). Baseline and extended follow-up reports of daytime sleepiness were each and classified into three groups: 1) never, 2) sometimes, and 3) often or almost always.
Cognitive Function
Cognitive assessments were conducted 1-4 times during follow-up during years 8-18 as part of the study follow-up protocol and participation of subsets of the cohort in ancillary studies (16). We used the most recent cognitive scores for the current evaluation (2018-2020). Staff were centrally trained and certified in administration of the standardized cognitive assessments and were masked to participant’s randomization status (23). The cognitive battery included the Rey Auditory Verbal Learning Test (RAVLT)(24), Digit Symbol Coding (DSC)(25), the Modified Stroop Color and Word Test (Stroop)(26), and the Trail Making Test Parts A&B (27). The Modified Mini-Mental Status Exam (3MS)(28) was used to assess global cognitive function. Test scores were standardized as z-scores which were averaged to derive a cognitive composite score (23). Trail Making Test scores were re-ordered so that higher values indicate better performance.
Other measures
Staff collected demographic and clinical characteristics including age, gender, race and ethnicity, education level, and smoking status at baseline. Weight was measured with digital scales. Diabetes treatments (insulin, sulfonylureas, other) were recorded at baseline. Hypertension was defined by treatment or measured blood pressure >140/90 mmHg. Baseline CVD included self-report of myocardial infarction, heart bypass surgery, coronary artery bypass graft, carotid endarterectomy, lower leg angioplasty, aortic aneurysm, congestive heart failure, or stroke. The Beck Depression Inventory (BDI)(29) was assessed annually until year 14. Subsequently, the Patient Health Questionnaire-9 (PHQ-9) (30) was administered to assess depressive symptoms. We dichotomized depressive symptoms, with BDI scores of ≥11 and PHQ-9 scores of ≥5. APOE ε4 status, a dementia risk factor, was determined for participants who provided consent (80% of women versus 86% of men, p<0.001), using TaqMan genotyping (rs7412 and rs429358)(31).
Analytic Design
Descriptive statistics were prepared by intervention group. Continuous variables were compared with t-tests and categorical variables compared with χ2 tests. Comparisons of continuous variables across three levels of daytime sleepiness were made using ANOVA. Categorical variables were derived to represent daytime sleepiness at baseline and follow-up 12-13 years later (2013-2014). The daytime sleepiness variable ranged from 1- 3 based on the categories as described above (i.e., never; sometimes; often/almost always). We compared individuals who were included in the analysis to those who were not included due to attrition or missing data.
Regression analysis assessed the association between daytime sleepiness in 2013-2014 and cognitive performance in 2018-2020 adjusting for: intervention arm, age, gender, race and ethnicity, education, baseline levels of daytime sleepiness, BMI, and hypertension. We adjusted for prior cognitive scores and depressive symptoms at baseline and as assessed concurrently with each cognitive measure. We did not use time-varying covariates for risk factors to allow the evaluation of the impact of the intervention. As prior work suggested heterogeneous effects of the intervention by subgroups (18, 32), we tested interactions by intervention arm, age (+/-65 at baseline), gender, race and ethnicity, APOE ε4 status, baseline BMI, and baseline history of CVD; we tested for an interaction by baseline depression as depression is associated with both cognition and daytime sleepiness.
Results
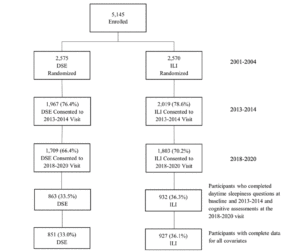
Participants who completed daytime sleepiness questions at baseline and proximal to the end of the intervention, and who completed cognitive evaluation at the most recent visit (n=1,778) were included in the analyses (Figure 1). A total of 3,367 participants were not included due either to being lost to follow-up or missing data for covariates of interest. These participants tended to be older, had lower levels of education, and included more APOE ε4 carriers; more of them had a baseline history of CVD, hypertension, insulin use, longer duration of diabetes, higher average HbA1c levels, and reported more depressive symptoms (Supplemental Table 1).
DSE=Diabetes Support and Education; ILI=Intensive Lifestyle Intervention
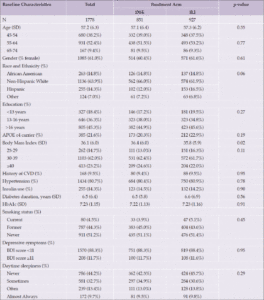
Among those included in the analysis, the average baseline age was 57 (standard deviation [SD] 6.3). The sample included more women (61%) than men, and was mostly White (63.9%) and highly educated (45.3% having college degree or higher). There were no significant differences by intervention arms among covariates listed in Table 1 except baseline BMI: the DSE group had a slightly higher average BMI (36.4 vs. 35.8 kg/m2) at baseline (p=0.02).
Abbreviations: APOE ε4=Apolipoprotein E gene, ε4 carrier status; BDI=Beck Depression Inventory; CVD=cardiovascular disease; DSE=diabetes support and education; ILI=intensive lifestyle intervention; SD=standard deviation.
Participant characteristics are reported in Table 2 by daytime sleepiness status at the 2013-2014 follow-up. A higher proportion of White participants reported often/always having daytime sleepiness and more Hispanic participants reporting sometimes having daytime sleepiness (p<0.001). Those with higher baseline BMIs reported more frequent daytime sleepiness (p=0.02). A higher proportion of participants with hypertension reported having daytime sleepiness sometimes as opposed to never or often/always (p=0.006). Depressive symptoms were more common among those who reported often/always having daytime sleepiness (p<0.001).
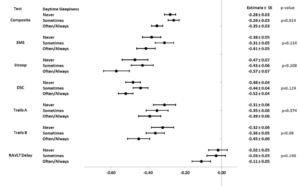
Figure 2 shows forest plots for least square means (±standard error) and p-values for associations between cognitive scores and frequency of self-reported daytime sleepiness. Models were adjusted for potential confounders including age, gender, race, ethnicity, education, randomization arm, and baseline values of BMI, hypertension, daytime sleepiness, and depressive symptoms. Models were also adjusted for 2013-2014 cognitive scores and concurrent assessments of depressive symptoms. Diabetes duration and smoking were considered but did not significantly contribute to the models and were dropped. Although the only statistically significant result is in the cognitive composite (p=0.014), forest plots demonstrate general dose-response effects such that participants reporting daytime sleepiness occurring often or always, had lower mean scores than those who reported lower levels of daytime sleepiness.
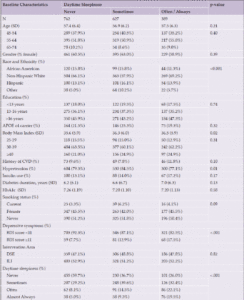
Table 2. Baseline Characteristics of 1,778 Look AHEAD participants by Daytime Sleepiness at 2013-2014
Abbreviations: APOE ε4=Apolipoprotein E gene, ε4 carrier status; BDI=Beck Depression Inventory; CVD=cardiovascular disease; DSE=diabetes support and education; ILI=intensive lifestyle intervention; SD=standard deviation.
Abbreviations: 3MS=Modified Mini-mental State Exam; DSC=Digit Symbol Coding; RAVLT Delayed=Rey Auditory Verbal Learning Test Delayed; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B. *Models are adjusted for treatment arm, the 2013-2014 value of the outcome, depressive symptoms at baseline, 2013-2014, and 2018-2020 visits, and baseline values of daytime sleepiness, age, gender, race and ethnicity, education, BMI, and hypertension.
In sensitivity analyses, we re-fitted models without controlling for cognitive scores at years 13-14 and similar but stronger associations between daytime sleepiness groups and some cognitive scores emerged (Supplemental Table 2). The composite z-score was in the same direction but lost significance (p=0.06), while the DSC (p=0.01) and Trails B (p=0.05) became significant when we did not control for prior cognitive scores.
We tested interactions by intervention arm, age, gender, race and ethnicity, APOE ε4 status, baseline BMI, history of CVD, and depression as suggested by prior work in Look AHEAD(16-18) and the literature on daytime sleepiness(33), using a p-value threshold of p=0.10. Associations between daytime sleepiness and cognitive scores varied across intervention groups (Table 3) with significant findings among those in the ILI group on DSC (p=0.05) and Trails B (p=0.02) such that those reporting daytime sleepiness often/always performed worse than the other two sleep groups (i.e., never or sometimes). We show similar results, albeit with stronger effects in Supplemental Table 3 which does not include adjustment for prior cognitive scores.
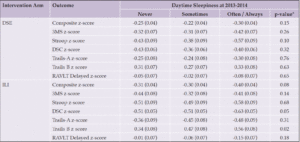
Table 3. Cognitive Performance in 2018-2020 by Daytime Sleepiness at 2013-2014 Stratified by Intervention Arm [LS Mean (SE)]
Abbreviations: 3MS=Modified Mini-mental State Exam; DSC=Digit Symbol Coding; RAVLT Delayed=Rey Auditory Verbal Learning Test Delayed; Trails A=Trail Making Test Part A; Trails B=Trail Making Test Part B. *Stratified models are adjusted for the 2013-2014 value of the outcome, depressive symptoms at baseline, 2013-2014 and 2018-2020 visits, and baseline values of daytime sleepiness, age, gender, race and ethnicity, education, BMI, and hypertension.
There were no significant interactions by age, gender, or baseline BMI. However, there were significant interactions between race and ethnicity categories and daytime sleepiness on nearly all our measures (shown in Supplemental Table 4). The trend across scores suggests lower performance on the composite (p=0.01), Stroop (p=0.03), DSC (p=0.02), Trails A (p=0.09) and Trails B (p=0.08), and RAVLT Delayed (p=0.07) with greater levels of daytime sleepiness. In most cases, African American and White participants demonstrated worse scores with more self-reported daytime sleepiness (sometimes or often/always). However, some apparent inconsistencies could be due to small numbers for groups including Hispanic participants and ‘other’ (which includes American Indian/Native American, mixed race, and others).
Interactions were found between daytime sleepiness and APOE ε4 status on cognitive scores on Trails A (p=0.05) and RAVLT Delayed (p=0.08) such that those who reported more daytime sleepiness and have one or more APOE ε4 allele(s), tended to perform worse than those without APOE ε4 allele(s). These interactions should be interpreted with caution however, because the number of participants with one or more APOE ε4 allele(s) is relatively small.
An interaction between baseline history of CVD and daytime sleepiness was apparent on the Stroop, with worse performance corresponding to higher levels of daytime sleepiness among those without a baseline history of CVD. Those who reported a baseline history of CVD and no daytime sleepiness performed worse than all the other groups (never: LS Mean= -0.69; p<0.01) on the Stroop. On the Trail Making Test Part A, participants with a baseline history of CVD demonstrated a trend toward worse performance with greater levels of self-reported daytime sleepiness (often/always: LS Mean=-0.68; p<0.08). On the Trail Making Test Part B, a similar trend was apparent with participants who had a baseline history of CVD performing worse with greater levels of daytime sleepiness (often/always: LS Mean=-0.63; p<0.09).
Finally, because there are established associations between depression and cognitive performance (34) as well as between depression and daytime sleepiness (35, 36), we tested for interactions between daytime sleepiness and depressive symptoms. Those with BDI score≥11 and no self-reported daytime sleepiness performed worse on DSC (never: LS Mean=-0.59) than those scoring <11; while those with BDI<11 and daytime sleepiness often or always performed nearly the same (LS Mean=-0.58). On Trails A, participants with BDI≥11 and reported daytime sleepiness sometimes (LS Mean=-0.59) and participants with BDI<11 reporting daytime sleepiness often/always (LS Mean=-0.43) performed worse than others (p<0.01). On Trails B the same pattern emerged where those with BDI≥11 and reported daytime sleepiness sometimes (LS Mean=-0.44) and participants with BDI<11 reporting daytime sleepiness often/always (LS Mean=-0.53) performed worse than others (p=0.02).
Discussion
We sought to test the degree to which self-reported daytime sleepiness was associated with the Look AHEAD intervention and cognitive scores. Participants who self-reported daytime sleepiness often or always in 2013-2014 performed significantly worse than those who reported sometimes or never having daytime sleepiness on the cognitive composite. Individual tests suggested a dose-response relationship, with greater levels of daytime sleepiness associated with worse performance. We further stratified by intervention arm, showing poorer scores on executive function tests were driven by the ILI group. This is aligned with prior reports showing no long-term cognitive benefit from the intervention (16). Randomization to ILI was not associated with self-reported daytime sleepiness, although it is feasible that any benefits accrued as a result of the intervention were subsequently lost over time.
To further probe drivers of these associations, we tested interactions based on prior work (16-18) and found differences by racial and ethnic groups on most tests, with African American and White participants demonstrating more consistent associations between greater levels of daytime sleepiness and poor performance on various tests compared to participants from Hispanic and Other (American Indian/Native American, mixed race, and others) groups. Participants with one or more APOE ε4 allele(s) and greater levels of daytime sleepiness performed worse on Trails A and RAVLT than non- APOE ε4 carriers. Poorer cognitive performance was observed among those with more frequent daytime sleepiness and a history of CVD compared to those with less frequent daytime sleepiness and no history of CVD. These results support earlier findings in Look AHEAD that suggested that participants reporting a baseline history of CVD experienced fewer cognitive benefits compared to others in the cohort (16). This result is also not surprising as CVD is associated with daytime sleepiness (37). Finally, tests for interactions with depressive symptoms showed consistent associations between higher daytime sleepiness levels and lower cognitive function among those reporting low levels of depression. However, among participants with greater levels of depressive symptoms, associations between daytime sleepiness and cognition were complex. This may be due to the bidirectional association between depression and disrupted sleep patterns (38).
Daytime sleepiness has been associated with adverse health outcomes in addition to CVD (37), including cognitive decline (5), cognitive impairment and dementia (7), as well as amyloid deposition (12, 39). In our study, daytime sleepiness was associated with poorer scores on the cognitive composite overall; on DSC and Trails B (executive function) among participants in the ILI group; and poorer scores on the Stroop, Trails A, and Trails B among those with a baseline history of CVD.
Our study has some limitations and strengths to note. Cognitive function was not measured at baseline as it was not a primary focus of the trial; therefore, we could not exclude participants based on cognitive impairment at baseline. However, our rigorous screening procedures effectively excluded those with clear impairment, and randomization facilitated comparable demographic and health characteristics across study arms at baseline. Therefore, there is no reason to suspect that the two groups would have differed in cognitive performance at baseline had it been measured. Our findings are generalizable to only a high-risk subset of the population, i.e., older adults with diabetes and overweight/obesity. However, this group, a growing segment of the population, has an increased risk of cognitive impairment, making this work valuable for individuals with diabetes and overweight/obesity. A strength of the work is the fact that Look AHEAD was a long-term randomized controlled clinical trial and was conducted using rigorous methods. Participants have been closely followed for nearly twenty years, providing deep phenotyping and well-characterized outcomes.
Our study adds to this body of literature by illustrating complex relationships between daytime sleepiness and cognitive performance among Look AHEAD participants who all had diabetes and overweight or obesity. Findings expand upon prior findings linking sleepiness to later amyloid deposition (12), and raise questions about the potential for daytime sleepiness (40) as an early indicator of cognitive decline, perhaps tied to atrophy in the locus coeruleus. Future longitudinal studies examining modifiable risk factors for dementia should include early measures of daytime sleepiness together with AD biomarkers to investigate these associations more thoroughly and determine their temporality. New targets for intervention that emerge early in the disease process are crucial to making advances in AD research.
Funding: Look AHEAD was funded by the National Institutes of Health through cooperative agreements with the National Institute on Aging and National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The National Institute of Aging provided funding for the Look AHEAD Mind ancillary study (R01AG058571) and the Look AHEAD Sleep ancillary (R01AG074562). MSPO was funded from R35HL155670. The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Acknowledgements: Clinical Sites: The Johns Hopkins University Jeanne M. Clark, MD, MPH1; Lee Swartz2; Dawn Jiggetts2; Jeanne Charleston, RN3; Lawrence Cheskin, MD3; Nisa M. Maruthur, MD, MHS3; Scott J. Pilla, MD, MHS3; Danielle Diggins; Mia Johnson; Pennington Biomedical Research Center George A. Bray, MD1; Frank L. Greenway, MD1; Donna H. Ryan, MD3; Catherine Champagne, PhD, RD3; Valerie Myers, PhD3; Jeffrey Keller, PhD3; Tiffany Stewart, PhD3; Jennifer Arceneaux, RN2; Karen Boley, RD, LDN2; Greta Fry, LPN; Lisa Jones; Kim Landry; Melissa Lingle; Marisa Smith2. The University of Alabama at Birmingham Cora E. Lewis, MD, MSPH1; Sheikilya Thomas, PhD, MPH2; Stephen Glasser, MD3; Gareth Dutton, PhD3; Amy Dobelstein; Sara Hannum; Anne Hubbell, MS; DeLavallade Lee; Phyllis Millhouse, L. Christie Oden; Cathy Roche, PhD, RN, BSN; Jackie Grant; Janet Turman. Harvard Center: Massachusetts General Hospital. David M. Nathan, MD1; Valerie Goldman, MS, RDN2; Linda Delahanty, MS, RDN3; Mary Larkin, MS, RN; Kristen Dalton, BS; Roshni Singh, BS; Melanie Ruazol, BS. Joslin Diabetes Center: Joslin Diabetes Center: Medha N Munshi, MD1; Sharon D. Jackson, CCRC, MS, RD, CDE2; Roeland J.W. Middelbeek MD3; A. Enrique Caballero, MD, Anthony Rodriguez. Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1*; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; University of Colorado Anschutz Medical Campus Holly Wyatt, MD1; James O. Hill, PhD1; Jeanne Anne Breen, MS2; Marsha Miller, MS, RD2; Debbie Bochert; Suzette Bossart; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Eugene Leshchinskiy; Loretta Rome, TRS. The University of Tennessee Health Science Center: University of Tennessee East. Karen C. Johnson, MD, MPH; Beate Griffin, RN, BS; Mace Coday, PhD3; Donna Valenski, Linda Jones; Karen Johnson, RN. University of Tennessee Downtown: Karen C. Johnson, MD, MPH; Beate Griffin, RN, BS; Helmut Steinburg, MD3. University of Minnesota: Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Mary Ann Forseth, BA; Soni Uccellini, BS; Mary Susan Voeller, BA. Columbia University Medical Center: Blandine Laferrère, MD, PhD1; Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Jose Luchsinger, MD1; Priya Palta, PhD,MHS3; Jennifer Patricio, MS2; Sarah Lyon, Kim Kelly. University of Pennsylvania: Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2; Robert I. Berkowitz, MD3; Ariana Chao, PhD, CRNP3; Renee Davenport; Katherine Gruber, CRNP; Sharon Leonard, RD; Olivia Walsh, BA. University of Pittsburgh: John M. Jakicic, PhD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lin Ewing, PhD, RN3; Andrea Hergenroeder, PhD, PT, CCS3; Mary Korytkowski, MD3; Susan Copelli, BS, CTR; Rebecca Danchenko, BS; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Lisa Martich, BS, RD, LDN; Meghan McGuire, MS; Tracey Y. Murray, BS; Linda Semler, MS, RD, LDN; Kathy Williams, RN, MHA. The Miriam Hospital/Brown Medical School: Rena R. Wing, PhD1; Caitlin Egan, MSv; Elissa Jelalian, PhD3; Jeanne McCaffery, PhD3; Kathryn Demos McDermott, PhD3; Jessica Unick, PhD3; Kirsten Annis, BA; Jose DaCruz; Ariana Rafanelli, BA. The University of Texas Health Science Center at San Antonio: Helen P. Hazuda, PhD1; Juan Carlos Isaac, CCRC, BSN2; Prepedigna Hernandez, RN. VA Puget Sound Health Care System / University of Washington: Steven E. Kahn, MB, ChB1; Edward J. Boyko, MD, MPH3; Elaine Tsai, MD3; Lorena Wright, MD3; Karen Atkinson, RN, BSN2; Ivy Morgan-Taggart; Jolanta Socha, BS; Heidi Urquhart, RN. Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico: William C. Knowler, MD, DrPH1; Paula Bolin, RN, MCv; Harelda Anderson, LMSWv, Sara Michaels, MD3; Ruby Johnson; Patricia Poorthunder; Janelia Smiley . University of Southern California: Anne L. Peters, MD1; Siran Ghazarian, MD2; Elizabeth Beale, MD3; Edgar Ramirez; Gabriela Rodriguez, MA; Valerie Ruelas MSW, LCSW; Sara Serafin-Dokhan; Martha Walker, RD; Marina Perez.
Coordinating Center: Wake Forest University Mark A. Espeland, PhD1; Kathleen Hayden, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH3; David Reboussin, PhD3; Nicholas Pajewski, PhD3; Jingzhong Ding, PhD3; Gagan Deep, PhD3; Stephen R. Rapp, PhD3; Bonnie C. Sachs, PhD3; Jerry M. Barnes, MA; Tara D. Beckner; Delilah R. Cook; Joni Evans, MS; Katie Garcia, MS; Sarah A. Gaussoin, MS; Carol Kittel, MS; Lea Harvin, BS; Marjorie Howard, MS; Rebecca H. Neiberg, MS; Jennifer Walker, MS; Michael P. Walkup, MS. 1Principal Investigator; 2Program Coordinator; 3Co-Investigator; All other Look AHEAD staffs are listed alphabetically by site.
Federal Sponsors: National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Robert Kuczmarski, DrPH; Rebecca Van Raaphorst, MPH; Susan Z. Yanovski, MD; National Institute on Aging Marcel Salive, MD, MPH.
Conflict of Interest Statement: Adam Spira received honoraria for serving as a consultant to Merck and from Springer Nature Switzerland AG for guest editing special issues of Current Sleep Medicine Reports. The other authors report no conflicts of interest.
Statement of Ethics: This study was conducted ethically, in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the Wake Forest School of Medicine (Look AHEAD study Coordinating Center) Institutional Review Board (IRB #BG99-042) as well as the Institutional Review Boards of all the data collection sites. All participants provided written informed consent to participate. The ClinicalTrials.gov identifier is NCT00017953.
Author Contributions: Drs. Hayden Spira, St-Onge, Ding, Molina-Henry, Sanderlin, Reboussin, Espeland and Ms. Anderson and Bahnson made substantial contributions to the design of this study. Drs. Reboussin, Espeland, and Ms. Anderson and Bahnson made substantial contributions for the collection of Look AHEAD data for this study. Drs. Espeland and Hayden made substantial contributions for the acquisition of Look AEHAD MIND data. Drs. Hayden and Espeland, and Ms. Anderson made substantial contributions to the analysis of the data for this study. All authors took part in drafting and revising this manuscript and gave final approval for this manuscript prior to submission.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. J Clin Psychiatry. 2004;65 Suppl 16:12-16.
2. Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of Daytime Sleepiness in 4578 Elderly Persons: The Cardiovascular Health Study. Sleep. 1998;21(1):27-36. https://doi.org/10.1093/sleep/21.1.27
3. Newman AB, Spiekerman CF, MD PE, et al. Daytime Sleepiness Predicts Mortality and Cardiovascular Disease in Older Adults. Journal of the American Geriatrics Society. 2000;48(2):115-123. https://doi.org/10.1111/j.1532-5415.2000.tb03901.x
4. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd ed. American Academy of Sleep Medicine; 2005.
5. Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. Sep 1 2012;35(9):1201-7. https://doi.org/10.5665/sleep.2070
6. Nakakubo S, Doi T, Makizako H, et al. Sleep condition and cognitive decline in Japanese community-dwelling older people: Data from a 4-year longitudinal study. J Sleep Res. Aug 2019;28(4):e12803. https://doi.org/10.1111/jsr.12803
7. Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. Dec 2001;49(12):1628-32. https://doi.org/10.1046/j.1532-5415.2001.t01-1-49271.x
8. Smagula SF, Jia Y, Chang CH, Cohen A, Ganguli M. Trajectories of daytime sleepiness and their associations with dementia incidence. J Sleep Res. Dec 2020;29(6):e12952. https://doi.org/10.1111/jsr.12952
9. Maestri M, Romigi A, Schirru A, et al. Excessive daytime sleepiness and fatigue in neurological disorders. Sleep Breath. Jun 2020;24(2):413-424. https://doi.org/10.1007/s11325-019-01921-4
10. Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. Mar 2009;10(3):211-23. https://doi.org/10.1038/nrn2573
11. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. Nov 2011;70(11):960-9. https://doi.org/10.1097/NEN.0b013e318232a379
12. Spira AP, An Y, Wu MN, et al. Excessive daytime sleepiness and napping in cognitively normal adults: associations with subsequent amyloid deposition measured by PiB PET. Sleep. Oct 1 2018;41(10):zsy152-zsy152. https://doi.org/10.1093/sleep/zsy152
13. Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. Aug 2005;90(8):4510-5. https://doi.org/10.1210/jc.2005-0035
14. Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea–a review. Sleep. May 1 2012;35(5):605-15. https://doi.org/10.5665/sleep.1812
15. Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. May 1 2013;36(5):641-649A. https://doi.org/10.5665/sleep.2618
16. Hayden KM, Neiberg RH, Evans JK, et al. Legacy of a 10-Year Multidomain Lifestyle Intervention on the Cognitive Trajectories of Individuals with Overweight/Obesity and Type 2 Diabetes Mellitus. Dement Geriatr Cogn Disord. 2021;50(3):237-249. https://doi.org/10.1159/000517160
17. Espeland MA, Rapp SR, Bray GA, et al. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69(9):1101-1108. https://doi.org/10.1093/gerona/glu031
18. Espeland MA, Luchsinger JA, Baker LD, et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. May 23 2017;88(21):2026-2035. https://doi.org/10.1212/WNL.0000000000003955
19. Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. Oct 2003;24(5):610-28. https://doi.org/10.1016/s0197-2456(03)00064-3
20. Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. Jul 11 2013;369(2):145-54. https://doi.org/10.1056/NEJMoa1212914
21. Look ARG, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). May 2006;14(5):737-52. https://doi.org/10.1038/oby.2006.84
22. The Look Ahead Research Group. The development and description of the comparison group in the Look AHEAD trial. Clinical Trials. 2011;8(3):320–329. https://doi.org/10.1177/1740774511405858
23. Rapp SR, Luchsinger JA, Baker LD, et al. Effect of a Long-Term Intensive Lifestyle Intervention on Cognitive Function: Action for Health in Diabetes Study. J Am Geriatr Soc. May 2017;65(5):966-972. https://doi.org/10.1111/jgs.14692
24. Rey A. L’examen clinique en psychologie. 1958;
25. Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III), The psychological corporation. San Antonio TX; 1997.
26. Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643. https://doi.org/10.1037/h0054651
27. Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Perceptual and Motor Skills. 2016;8(3):271-276. https://doi.org/10.2466/pms.1958.8.3.271
28. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. Aug 1987;48(8):314-8. PMID: 3611032
29. Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. Beck depression inventory (BDI). Arch Gen Psychiatry. 1961;4(6):561-571. https://doi.org/10.1001/archpsyc.1961.01710120031004.
30. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. Sep 2001;16(9):606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
31. Elosua R, Demissie S, Cupples LA, et al. Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obes Res. Dec 2003;11(12):1502-8. https://doi.org/10.1038/oby.2003.201
32. Espeland MA, Carmichael O, Yasar S, et al. Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimers Dement. Sep 2018;14(9):1184-1192. https://doi.org/10.1016/j.jalz.2018.05.015
33. Fernandez-Mendoza J, Vgontzas AN, Kritikou I, Calhoun SL, Liao D, Bixler EO. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep. Mar 1 2015;38(3):351-60. https://doi.org/10.5665/sleep.4488
34. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. Dec 2009;119(1-3):1-8. https://doi.org/10.1016/j.jad.2009.04.022
35. Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiat. 2004;65(Suppl 16):27-32. PMID: 15575802
36. Chellappa SL, Schröder C, Cajochen C. Chronobiology, excessive daytime sleepiness and depression: Is there a link? Sleep medicine. 2009;10(5):505-514. https://doi.org/10.1016/j.sleep.2008.05.010
37. Wang L, Liu Q, Heizhati M, Yao X, Luo Q, Li N. Association between Excessive Daytime Sleepiness and Risk of Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. J Am Med Dir Assoc. Dec 2020;21(12):1979-1985. https://doi.org/10.1016/j.jamda.2020.05.023
38. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. Apr 2019;23(4):2324-2332. https://doi.org/10.1111/jcmm.14170
39. Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. Sep 2015;36(9):2568-76. https://doi.org/10.1016/j.neurobiolaging.2015.05.004
40. Plini ERG, O’Hanlon E, Boyle R, et al. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells. Jul 20 2021;10(7) https://doi.org/10.3390/cells10071829
© The Authors 2023