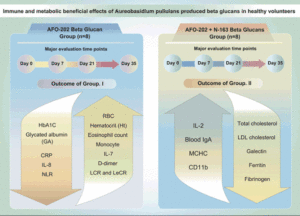
N. Ikewaki1,2, T. Sonoda2, G. Kurosawa3,4, M. Iwasaki5, V. Devaprasad Dedeepiya6, R. Senthilkumar7,8, S. Preethy7, S.J.K. Abraham5,6,8,9,10
1. Dept. of Medical Life Science, Kyushu University of Health and Welfare, Japan; 2. Institute of Immunology, Junsei Educational Institute, Nobeoka, Miyazaki, Japan;
3. Department of Academic Research Support Promotion Facility, Center for Research Promotion and Support, Fujita Health University, Aichi, Japan; 4. MabGenesis KK, Nagoya, Japan; 5. Centre for Advancing Clinical Research (CACR), University of Yamanashi – School of Medicine, Chuo, Japan; 6. Mary-Yoshio Translational Hexagon (MYTH), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India; 7. Fujio-Eiji Academic Terrain (FEAT), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India; 8. Antony- Xavier Interdisciplinary Scholastics (AXIS), GN Corporation Co. Ltd., Kofu, Japan; 9. R & D, Sophy Inc., Japan; 10. Levy-Jurgen Transdisciplinary Exploratory (LJTE), Global Niche Corp, Wilmington, DE, USA
Corresponding Author: Dr. Samuel JK Abraham, University of Yamanashi – School of Medicine, Chuo, Japan, 3-8, Wakamatsu, Kofu, Yamanashi 400-0866, Japan. Email id- drsam@nichimail.jp ; Alternate email id: drspp@nichimail.jp, Phone: +81-55-235-7527
J Aging Res & Lifestyle 2023;12:61-71
Published online July 28, 2023, http://dx.doi.org/10.14283/jarlife.2023.11
Abstract
OBJECTIVES: In this pilot study, we have evaluated the specific metabolic and immune-related benefits of the AFO-202 strain and N-163 strain of black yeast Aureobasidium pullulans-produced beta 1,3-1,6 glucan in healthy human subjects.
METHODS: Sixteen healthy Japanese male volunteers (aged 40 to 60 years) took part in this clinical trial. They were divided into four groups (n = 4 each): Group I consumed AFO-202 beta-glucan (2 sachets of 1 g each per day), IA for 35 days and IB for 21 days; Group II consumed a combination of AFO-202 beta-glucan (2 sachets of 1 g each) and N-163 beta-glucan (1 sachet of 15 g gel each per day), IIA for 35 days and IIB for 21 days.
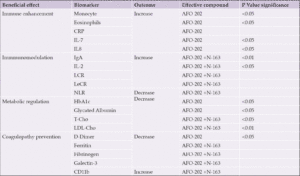
RESULTS: Decrease in HbA1C and glycated albumin (GA), significant increase of eosinophils and monocytes and marginal decrease in D-dimer levels, decrease in neutrophil-to-lymphocyte ratio (NLR), with an increase in the lymphocyte-to-CRP ratio (LCR) and leukocyte-to-CRP ratio (LeCR) was observed in Group I between pre- and post-treatment. Decrease in total and LDL cholesterol, a decrease of CD11b, serum ferritin, galectin-3 and fibrinogen were profound in Group II between pre- and post-treatment. However, there was no statistically significant difference between day 21 and day 35 among the groups.
CONCLUSION: This outcome warrants larger clinical trials to explore the potentials of these safe food supplements in the prevention and prophylaxis of diseases due to dysregulated metabolism, such as fatty liver disease, and infections such as COVID-19 in which balanced immunomodulation are of utmost importance, besides their administration as an adjunct to existing therapeutic approaches of both communicable and non-communicable diseases.
Key words: AFO-202, N-163 strains of black yeast, Aureobasidium pullulans, beta glucans, immune enhancement, immunomodulation, glucotoxicity, lipotoxicity, metabolism, COVID-19, fatty liver disease.
Introduction
Metabolism imbalance is a gradually occurring condition leading to diabetes, heart disease, stroke, etc., and the risk varies between populations based on their genetic predisposition, diet, lifestyle, and environmental influences (1). When an individual is diagnosed with any lifestyle illness requiring medication, further prevention and deceleration of the pathogenesis is an uphill task. To address this, exercise, dietary modifications such as intake of foods with low glycaemic index or fats, and medications are advised which are temporary and not definitive solutions (1). This study aims to study the effects of Aureobasidium pullulans AFO-202 and N-163 strains-produced biological response modifier beta glucan food supplements in middle-aged, healthy subjects. The rationale behind this objective is explained below.
The liver, being the metabolic centre of the body, often becomes the key target (2), and coronary artery and cerebral systemic coagulopathies (1) may also occur. Additionally, the immune reserves may be depleted in handling, and the circulating high levels of advanced glycation end products (AGEs) and lipids affect the functional capability of the immune cells, leading to high risk of disease severity (3) when COVID-19-like infections occur (4). The fibrosis that ensues after a chronic inflammation-metabolic-immune dysregulation can lead to pulmonary or liver fibrosis, such as non-alcoholic steatohepatitis (NASH) (2), which could eventually culminate in carcinogenesis (5). Glucotoxicity and lipotoxicity also cause gut dysbiosis (6), which is now increasingly considered the key factor influencing the progression of infections, inflammations, and fibrosis, creating a vicious cycle.
Against the given background, as a remedy, what we require is an agent which should be safe and possesses the following potentials.
Regarding glucotoxicity and lipotoxicity
At an early stage or before onset of disease
It should be able to balance the blood glucose levels, especially the post-prandial spike and balance the blood cholesterol level without any side effects.
Post-disease onset stage
During and after onset of the glucotoxicity and/or lipotoxicity, it should be able to control abnormal glucose and cholesterol levels without any side effects and without any adverse interactions with other drugs prescribed. It should be able to beneficially regulate LDL and VLDL without adversely affecting HDL and should be able to control inflammation and the accumulation of free fatty acids (FFA).
After progression of disease with chronic sequalae stage
It should be able to control organ inflammatory reaction to avoid fibrosis and also balance micro-inflammation of the gut.
Regarding systemic wellness and immune balance, throughout the various stage, mentioned above, it should support the immune system, especially during aging, by enhancing it to prevent illnesses from disease-affected weakness. It should be able to promote immune modulation to avoid hyper-activation and cytokine storm. It should have potential to balance immune enhancement and modulation to avoid pre-disposing factors to carcinogenesis and also reverse gut dysbiosis.
Although a single such prophylactic measure or component is almost impossible, we selected two products of strains from the black yeast A. pullulans which have a track record of safety (7-9) and potential to restore the gut microbiome (10, 11).
AFO-202 benefits
The AFO-202 strain-produced beta glucan has been shown to normalize Hba1c and fasting, post-prandial blood glucose levels in patients with type II diabetes (7). It has been shown to decrease elevated LDL and VLDL cholesterol and triglycerides in clinical studies of metabolic syndrome (8). Enhancement of immune cells such as natural killer (NK) cells and macrophages, apart from suppression of pro-inflammatory cytokines while enhancing beneficial cytokines and antibodies has been reported (9). Apart from these beneficial immune and metabolic modulations, a decrease in the neutrophil-to-lymphocyte ratio (NLR) and increase in lymphocyte-to-C-reactive protein (CRP) ratio (LCR) and leukocyte-to-CRP ratio (LeCR) are particularly significant in COVID-19 (12), as the dysregulation of these parameters has been correlated with progression of the disease and higher odds of mortality (13).
N-163 benefits
While AFO-202 is relevant to both metabolic and immune regulation, the anti-inflammatory, anti-fibrotic potential of N-163 has been reported with significance in a NASH animal model (14), along with a decrease in inflammation-associated lipid parameters such as non-esterified free fatty acids (NEFAs) (15). Thus, N-163 is more relevant in the stages of progressed disease status.
The potential of the AFO-202 and N-163 beta glucan as an immune adjuvant in the prophylaxis of COVID-19, along with beneficial anti-coagulopathy benefits in clinical trials, has been described (16-20). These beta glucans have also been effective as inti-infective agents against viral infections such as dengue, influenza, rabies apart from beneficial immune-modulation in sepsis (21-24).
Before addressing specific disease targets, we sought to study the effects of AFO-202 and N-163-produced beta glucans in the middle-aged, healthy subjects, as they have been the most vulnerable population for metabolic diseases (25) and severe COVID-19.
Methods
The study was conducted in compliance with the ethical principles based on the Declaration of Helsinki. The study protocol was approved by the institutional review board (IRB) of Chiyoda Paramedical Care Clinic, Tokyo, Japan (study protocol number GNC20C1), and registered with the University Hospital Medical Information Network-Clinical Trial Registry (UMIN-CTR) of Japan, Trial registration Number UMIN: 000040882 (26). The study was conducted at the Chiyoda Paramedical Care Clinic, Tokyo, Japan.
Patient and Public involvement
The subjects and the public were involved in the design and conduct of this research. During the feasibility stage, priority of the research question, choice of outcome measures, and methods of recruitment were informed by discussions with the subjects through a focus group session and structured interviews. Once the trial is published, participants will be informed of the results through a study newsletter suitable for a non-specialist audience.
Study Subjects
The study was designed as an exploratory study in sixteen healthy Japanese male volunteers aged 40 to 60 years with four intervention conditions: two test food groups and two durations of intake in each test food group.
The person in charge of the allocation, as specified in the study protocol, allocated the study subjects to the four groups as evenly as possible, giving first priority to pre-test BMI, second priority to weight, and third priority to height.
Subjects who met the selection criteria (26) and did not fall under any of the exclusion criteria were eligible for the study.
The CONSORT flow diagram of the study is available in the supplementary material.
Intervention
The duration of the study food intake and the schedule of visits for each group was:
Group I
AFO-202 beta glucan (1g containing 42 mg active ingredient) – 2 sachets with each meal
• IA: Intervention for 35 days
• IB: Intervention for 21 days
Group II
AFO-202 beta glucan (1g containing 42 mg active ingredient) – 2 sachets with each meal + N-163 (15 g gel sachet containing 90 mg of the active ingredient) – 1 sachet with any one of the meals
• IIA: Intervention for 35 days
• IIB: Intervention for 21 days
Primary endpoints
1. Immune activation effect
2. WBC, RBC, Hb, Ht, PLT, MCV, MCH, MCHC
3. Basophils, eosinophils, neutrophils, lymphocytes, monocyte counts
4. CRP, IgG in blood, IgM in blood, IgA in blood
5. IL-2, IL-6, IL-7, IL-8, IFN-γ, sFas ligand
Secondary endpoints
1. Coagulopathy related markers
i. Ferritin, D-dimer, PT, Fib, CD11b in monocyte fraction, galectin-3
2. Blood glucose level
3. HbA1c, GA
4. Cholesterol level
i. TG, T-Cho, HDL-Cho, LDL-Cho
Safety evaluation items
Incidence of adverse effects.
Evaluations
At pre-test and before intake
Blood sampling volume: 34 mL
Background survey was performed to gather information on the gender, date of birth, age, smoking habits, drinking habits, eating habits, current medical history, medication, treatment, previous history, allergies (to drugs and food), regular use of food for specified health uses, functional foods, health foods, intake of foods rich in β-glucan foods containing beta-glucan, intake of immunity-boosting foods, and blood donation (within 1 year).
The following assessments were performed,
– Medical history and physical measurements: medical history, height, weight, BMI, temperature
– Physiological examination: systolic blood pressure, diastolic blood pressure, pulse rate
– Haematology, cellular immunology assessments and blood biochemistry
Day 21 of intake and Day 35 of intake
– Blood collection volume: 31 mL
– History and physical examination: history, weight, BMI
– Physiological examination: systolic blood pressure, diastolic blood pressure, pulse rate
– Haematology, cellular immunology assessments and blood biochemistry
Daily diary
The diary was maintained from the day of the start of the consumption of the test food until the 35th day of consumption. The following items were recorded in the diary.
Intake of test foods, body temperature, intake of food for specified health uses, functional foods, and health foods, intake of restricted foods, subjective symptoms, visits to medical institutions, treatment, and use of medicines.
Examples of restricted foods
Supplements rich in beta-glucan: supplements containing beta-glucan extracted and concentrated from yeast, barley, mushrooms and seaweed.
Foods claiming to stimulate the immune system: yoghurt, lactobacillus beverages, bifidobacteria powder, propolis, lactoferrin, etc.
Statistical analysis
The statistical significance level was set at 5%, two-sided. SPSS26.0 (IBM Japan, Ltd.) and Microsoft Excel (Microsoft Corporation) were used as analysis software. An unpaired t-test, Fisher’s exact test (Bonferroni correction), Dunnett certification, and a correspondence t-test were performed.
Results
One study subject (No. 4) with leukocyte abnormalities (suspected leukaemia) discontinued or dropped out of the study. In addition, two study subjects (Nos. 11 and 16) were excluded because they fell under “6) Other obvious reasons for omission” in the “Exclusion criteria for PPS analysis” (26) section. After excluding these two subjects from the FAS, 13 subjects were included in the PPS.
Comparisons between the test food groups using the change from pre-consumption values showed statistically significant differences in the parameters outlined in Table 1.
Abbreviations: CRP- C-reactive protein; IL-Interleukin; Ig-Immunoglobin; LCR-Lymphocyte to C-reactive protein ratio; NLR- Neutrophil to Lymphocyte ratio; LeCR- Leukocyte to C-reactive protein ratio; LDL-Low density lipoprotein
AFO-202 beta glucan
Glucose metabolism
HbA1C
In Group I, the decrease post-intervention was greater by -0.23 ± 0.06% after 35 days of intake compared with Group II (-0.08 ± 0.05%), which showed a statistically significant higher value (p < 0.05) (Figure 1A).
Glycated albumin (GA)
After 21 days of consumption, the GA decrease in Group I (-0.53 ± 0.15%) was statistically significantly higher than that of the Group II (-0.10 ± 0.18%) (p < 0.05), (Figure 1B).
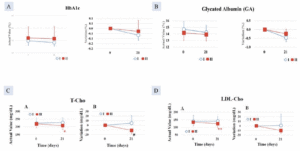
Figure 1. Decrease in A. HbA1c; B. Glycated albumin (GA); significantly greater in Group I (AFO-202 beta glucan) compared to Group II (AFO-202+N-163 beta glucan); Decrease in C. total cholesterol (T-Cho) and; D. LDL-cholesterol significantly greater in Group II (AFO-202+N-163 beta glucan) compared to Group I (AFO-202 beta glucan)
Haematological indices of immune stimulation
RBC
After 21 days of consumption, the RBC was statistically significantly higher (p < 0.05) in Group I (4.0 ± 5.3 x 104/µL) compared with test Group II (-8.8 ±5.6 x 104/µL).
Hb
After 21 days of consumption, the value in Group I (0.13 ± 0.12 g/dL) (p < 0.01) was statistically significantly higher compared with that of Group II (-0.38 ± 0.15 g/dL). Haematocrit (Ht)
After 21 days of intake, Group I (-0.03 ± 0.40%) showed statistically significant higher Ht values than did Group II (-1.50 ± 0.29%) (p < 0.01).
Eosinophils
A statistically significant difference was found between the test food groups in terms of the change from pre-treatment to post-treatment (p < 0.05). Eosinophil count (0.50 ± 0.54%) was higher in Group I compared with Group II (-0.36 ± 0.61%) (Figure 2A).
Monocytes
After 7 days of consumption, Group I (6.63 ± 0.51%) showed a statistically significantly higher monocyte value than did Group II (5.00 ± 0.82%) (p < 0.05). After 21 days of consumption, Group I (1.93 ± 0.47%) also showed a statistically significant increase compared with Group 2 (0.87 ± 0.21%) (p < 0.05) (Figure 2B).
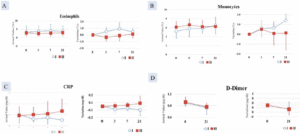
Figure 2. Increase in A. Eosinophil count; B. Monocytes count significantly greater in Group I (AFO-202 beta glucan) compared to Group II (AFO-202+N-163 beta glucan); decrease in C. CRP and D. D-Dimer, significantly greater in Group I (AFO-202 beta glucan) compared to Group II (AFO-202+N-163 beta glucan)
CRP
At 21 days, the decrease in CRP was greater in Group I (level= 0.0517 mg/dl) compared with Group II (0.1329 mg/dl), which was statistically significant (p < 0.05) (Figure 2C).
IL-7
After 7 days of consumption, the IL-7 level was statistically significantly higher (p < 0.05) in Group I (4.33 ±0.87 pg/mL) compared with Group II (2.67 ±0.55 pg/mL).
IL-8
Group I’s IL-8 values (7.003 ±0.929 pg/mL) were statistically significantly higher than those of Group II (5.230 ±0.469 pg/mL) after 7 days of intake (p < 0.05).
D-dimer
After 35 days of intake, the D-dimer decrease in Group I (-0.30 ±0.10 µg/mL) was statistically significantly higher than that of the test food Group II (0.00 ±0.10 µg/mL) (p < 0.05) (Figure 2D).
NLR, LCR, and LeCR
The decrease in NLR was greater in Group I at day 21, but at day 35, the decrease was higher in Group II. In terms of LCR and LeCR, at day 35, the increase from baseline value was greater in Group I compared with Group II (Figure 3A-C). The results however were not statistically significant.
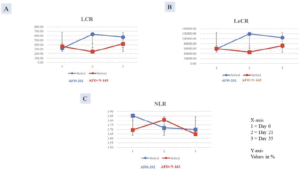
Figure 3. Increase in A.LCR; B. LeCR and decrease in C. NLR significantly greater in Group I (AFO-202 beta glucan) compared to Group II (AFO-202+N-163 beta glucan)
N-163
Regulation of lipid parameters
Total cholesterol (T-Cho)
After 21 days of intake, the T-Cho decrease in Group II (-12.8 ± 4.0 mg/dL) was statistically significantly higher than that of the test food Group I (9.0 ± 12.3 mg/dL) (p < 0.05) (Figure 1C).
LDL cholesterol (LDL-Cho)
There was a statistically significant decrease in LDL-Cho in Group II, at 124.0 ±25.3 mg/dL, after 21 days of consumption, compared with 134.0 ±25.2 mg/dL before consumption (p < 0.01) (Figure 1D).
Immuno-modulation and anti-inflammatory effects
IL-2
The increase to 0.3743 ± 0.1165 pg/mL after 14 days of post-observation in Group II was statistically significant higher (p < 0.05) than the 0.1220 ± 0.0635 pg/mL value in Group 1.
Blood IgA
After 21 days of intake, Group II (340.3 ±64.9 mg/dL) had a statistically significantly higher blood IgA value than did Group I (175.0 ±9.5 mg/dL) (p < 0.01) (Table 1).
MCHC
After 7 days of consumption, the MCHC was statistically significantly higher (p < 0.05) in Group II (32.56 ± 0.55%) compared with Group I (31.85 ± 0.55%).
Serum galectin, ferritin, and fibrinogen
The decrease in serum fibrinogen, ferritin and galectin-3 was greater in Group II compared with Group I, but the difference was not significant (Figure 4A-C).
CD11b
An increase in CD11b in the monocyte fraction was observed in Group II after 21 days of ingestion compared with Group I but it was not statistically significant (Figure 4D).
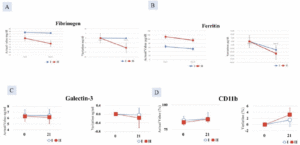
Figure 4. Decrease in A. Fibrinogen and; B. Ferritin C. Galectin-3 and D. increase in CD11b greater in Group II (AFO-202+N-163 beta glucan) compared to Group I (AFO-202 beta glucan)
Other parameters
No statistically significant difference was observed in the other parameters. There was no discernible difference in the food consumption practices in the individuals, post-intervention.
Safety endpoints (incidence of adverse reactions)
No adverse reactions occurred in this study.
Discussion
The results of the study has proven the hypothesis suggest that A.pullulans produced beta glucans exert beneficial metabolic and immune effects, with the AFO-202 beta glucan capable of eliciting beneficial effects in balancing blood glucose, alleviating glucotoxicity with immune activation, while a combination of AFO-202 and N-163 beta glucans has significant anti-inflammatory and lipid profile regulating potential, thereby alleviating lipotoxicity.
Metabolic syndrome (MeTS) is a significant health issue in today’s world, affecting one quarter of the global population, which amounts to over a billion people (27). Although lifestyle changes remain the primary modality of therapy, several drugs, including statins and anti-diabetic medications, are major agents used in therapy, which do little to treat the secondary symptoms and are associated with side effects (28, 29).
In addition, these therapeutic approaches focus on either glucotoxicity resulting from irregular and unmanageable blood glucose levels, or lipotoxicity caused by an imbalanced lipid profile. However, they do not tackle the immune system disturbances triggered by MeTS (1, 4). Advancing metabolic disruption, enhanced by aging-induced inflammatory disorders (inflammaging), leads to accumulation of lipids in the aging organs, coupled with immunosenescence (3) which further increases individuals’ risk of contracting infectious diseases. It is important to note that therapeutic strategies are used after the disease has already started and are not given as a preventative measure.
A continuous safe supplementation approach, which could serve as a prophylaxis before the onset of disease and an adjunct to existing treatments after disease onset, could be a holistic solution for which the A. pullulans’ novel strains-produced exopolysaccharide beta glucan-based biological response modifiers could be of potential use. The A. pullulans is a polyextremotolerant generalist black yeast belonging to the phylum Ascomycota, class Dothideomycete and order Dothideales having high levels of genetic recombination (30). The AFO-202 strain of this black yeast produced beta glucan, having been documented to alleviate glucotoxicity and enhance immunity (8-10), when combined with the N-163 strain produced beta glucan has significant balancing effects on the lipid profile, anti-inflammatory and anti-fibrotic effects with immunomodulation (12, 14-16), are further substantiated by the results of the present study.
In the present study in healthy Japanese men, AFO-202 has been shown to enhance the immune system, as observed from the increase in eosinophils and monocytes. A decrease in CRP observed in Group I (Figure 2C) with CRP being an acute phase reactant (31) and known to increase rapidly with the onset of cell injury and inflammation shows that the AFO-202 beta glucan has anti-inflammatory and immune enhancement potential. A significant increase in CD11b in the monocyte fraction was observed after 21 days of ingestion (Figure 4D). CD11b is expressed on monocytes, macrophages, dendritic cells, granulocytes, and NK cells and is an LPS receptor (32). It is associated with the bacteriophagocytic activity of phagocytes. The increase in CD11b in the monocyte fraction of Group II after 21 days of consumption compared with before can be considered as a manifestation of immune activation by N-163 beta glucan. There was no change in IgG or IgM levels in the blood throughout the study period in both test groups, but an increase in IgA levels along with the decrease in D-dimer by AFO-202 and of galectin, fibrinogen, and ferritin by the combination of AFO-202 and N-163 beta glucan (Group II) offers evidence in favour of the combined approach for addressing immune associated coagulopathy-associated risks in diseases such as COVID-19, in which a hyperactivated immune response affects the clotting pathway (13). The decrease in NLR with an increase in LCR and LeCR, all having been reported to be potential biomarkers of the underlying inflammation and hyperactivated immune response in COVID-19 (13), further substantiate the potent anti-inflammatory potential of these beta glucans. In terms of the secondary endpoints of glycaemic control and normalization of cholesterol levels, a longitudinal comparison showed a significant decrease in HbA1c and GA after 21 days of consumption in Group I and in T-Cho and LDL-Cho in Group II, suggesting that the synergistic intake of these beta glucans suppresses the increase in blood glucose level and lowers the cholesterol level, which could be useful in the context of metabolic disorders with underlying immune dysregulation, which again is a key factor associated with disease severity and mortality in COVID-19 (13), apart from application in management of MeTS and associated cardiac/cardiovascular abnormalities (33, 34). Though some of the parameters showed only a minor difference in values, the multi-system effects of these beta glucans are the main reason for recommending their use for health and wellness. The proposed mechanisms by which the AFO-202 and N-163 beta glucans produce beneficial effects include their recognition as pathogen-associated molecular patterns (PAMPs), through which they modulate the function of immune cells (35). The cytochrome P450 enzyme 7-hydroxylase (CYP7A1) catalyses the formation of primary bile acids and thereby regulates cholesterol synthesis and liver cholesterol excretion. Beta glucan regulates CYP7A1 and HMG-CoA, which in turn regulate cholesterol synthesis and its decomposition into bile acids. By regulating enzyme activity in the liver, the lipogenic effects of beta-glucans are elucidated (36, 37). Some of the metabolic effects of the beta glucans may also be mediated by the gut microbiota. As beta-glucans are resistant to digestion by gastric and pancreatic enzymes, they are fermented by the host’s microbiome in the colon and exert their effects in this manner. Viscosity-dependent health benefits of highly viscous fibres such as beta glucans also contribute to cholesterol-lowering and improved glycemic control. Through short-chain fatty acid (SCFA)-induced production of gut hormones, beta-glucan suppresses appetite and increases insulin sensitivity. Gastric emptying peptide and GLP-1 are hypothesised to be related to these alterations (38). Immune-mediated effects of glucan are primarily induced by pattern recognition receptors (PRRs). These include Dectin-1, CR3, TLRs, lactosylceramides, and scavenger receptors. Dectin-1 is the key beta glucan receptor. The recognition and binding of TLR and Dectin-1 control the immune response by modulating the release of pro- and anti-inflammatory cytokines (39). A so far unidentified beta-glucan receptor that induces an Akt/P13K-dependent anti-inflammatory response also contributes to the metabolic and immune effects [40]. Beta glucan is also a potent inducer of epigenetic and functional reprogramming of innate immune cells, a process known as «trained immunity» that improves the host’s response to infections (41). Beta glucan induces acquired immunity via histone modifications at gene promoters in human monocytes, which is accompanied by increased production of proinflammatory cytokines in response to a microbial challenge. The expansion of hematopoietic stem and progenitor cells in the bone marrow and the increase in myelopoiesis provide significant protection against infection. This protective signature of beta glucan is mediated by IL-1 signalling. Beta-glucans activate a variety of immune cells, including macrophages, neutrophils, monocytes, natural killer cells, and dendritic cells, by binding to immune receptors such as Dectin-1, complement receptor 3 (CR3), and TLR-2/6 (42). Beta glucans can also modulate the tumour microenvironment by bridging the innate and adaptive arms of the immune system and by altering the phenotype of immunosuppressive cells to make them immune-stimulatory, contributing to the effects against cancer (42).
Two critical areas of further research are essential for a holistic understanding of their benefits. One is the process of aging, against which all mechanisms must act, as aging is an inevitable phenomenon causing gradual loss of optimal functioning capability of the whole human body, from the cellular to organ level, and age-related cumulative pathogenesis, especially the immune system. The second essential component of research must be on the gut microbiota, also called the “second genome” (43), as their involvement and contribution to both metabolism balancing and immune modulation, besides neuronal implications for aging apoptosis, chronic micro-inflammation, and carcinogenesis (44), have been gaining strong evidence in the past decade. With the earlier studies of immune cell enhancement in young healthy volunteers (45) and elderly cancer patients (46) having been earlier proven as well with these beta glucans, a large population involvement to document the same would strengthen such findings. Also, the effects of the beta glucan supplementation on the food dynamics of the participants, post-intervention needs further research. As beta glucans are known for their pre-biotic effects, and their beneficial effects could be proven to correlate with the gut microbiota, we should be able to see an amalgamation of all these and their mechanisms of interaction to explain technically their various potentials in terms of prevention, prophylaxis, and as a therapeutic adjunct for both communicable and non-communicable diseases.
It an important limitation is that this study was performed in healthy volunteers as an exploratory study, which warrants further validation in translational models designed for specific diseases to confirm the efficacy in specific pathogenesis and in human clinical studies in target illnesses. Further, because these beta glucans have been in consumption for long, AFO-202 since 1996 and N-163 since 2018 with safety track record as a food supplement, apart from pre-clinical and clinical studies (7, 8, 10-12, 15), we did not include a control group, as the main objective was to compare the immunological benefits of AFO-202 beta glucan alone versus when it is combined with N-163 beta-glucan. Although the study sample was small, this study paves the way for future research on the effects of these safe nutritional supplements in prophylaxis and prevention of disease in high-risk individuals, such as those with MeTS, as well as in infectious and non-infectious immune-metabolic dysfunction-associated diseases such as COVID-19.
Conclusion
In summary, this study has demonstrated that the AFO-202 beta glucan is capable of eliciting beneficial effects in balancing blood glucose, alleviating glucotoxicity with immune activation, while a combination of AFO-202 and N-163 beta glucans has significant anti-inflammatory and lipid profile regulating potential, thereby alleviating lipotoxicity. Therefore, these beta glucans, being safe to consume, may be incorporated as a new beneficial adjunct therapy for individuals with developing and established MeTS. Future studies in subjects with relevant illnesses are warranted to evaluate these beta glucans’ application as agents for prophylaxis and management of fibrosis-induced non-communicable diseases such as fatty liver disease and immune hyperactivation-related diseases, especially in communicable diseases such as COVID-19. Though the factors capable of determining a reduction in severity and mortality are different and their interactions are not yet fully understood, further elaborate research on evaluation of these beta glucans for their pre-biotic potentials in gut microbiota and related outcomes in managing chronic microinflammation, apoptotic mechanisms, and carcinogenesis could lead to evolution of knowledge and henceforth applications.
Acknowledgement: The authors wish to acknowledge: a. M/s CPCC Co. Ltd. and M/s Chiyoda Paramedical Care Clinic, Tokyo, Japan for their assistance in planning and execution of the entire study. b. Dr. Mitsuru Nagataki and Late Mr. Takashi Onaka, (Sophy Inc, Kochi, Japan), for necessary technical clarifications. c. Dr. Kadalraja Raghavan, Research & Development Division, Sarvee Integra Pvt Ltd, Chennai, India and Dr. Vaddi Suryaprakash, Department of Urology, Yashoda Hospitals, Hyderabad, India for their technical inputs. d. Ms. Yoshiko Amikura of GN Corporation, Japan for their liaison assistance with the conduct of the study. e. Loyola-ICAM College of Engineering and Technology (LICET) for their support to our research work.
Ethics Approval: The study protocol was approved by the institutional review board (IRB) of Chiyoda Paramedical Care Clinic, Tokyo, Japan (Study protocol number GNC20C1) and registered with the University hospital Medical Information Network- Clinical Trial Registry UMIN-CTR of Japan (Ref No UMIN: 000040882: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000046681). The study was conducted at the Chiyoda Paramedical Care Clinic, Tokyo, Japan.
Sources of Support: No external funding was received for the study.
Author Declarations: Author Samuel Abraham is a shareholder in GN Corporation, Japan which in turn is a shareholder in the manufacturing company of novel beta glucans using different strains of Aureobasidium pullulans. The other authors don’t report any potential conflict of interests.
Author Contributions: CRediT author statement: Nobunao Ikewaki and Vidyasagar Devaprasad Dedeepiya; Conceptualization and Investigation: Rajappa Senthilkumar; Formal analysis: Sonoda, Gene Kurosawa and Masaru Iwasaki; Reviewing and editing: Senthilkumar Preethy; Writing original draft: Samuel JK Abraham; Conceptualization and writing original draft.
Data Availability: All data generated during the study are available in the manuscript itself.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215-225. doi: 10.1177/1753944717711379.
2. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-61. doi: 10.1016/j.metabol.2016.02.014
3. Moldogazieva NT, Mokhosoev IM, Mel’nikova TI, Porozov YB, Terentiev AA. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2019;2019:3085756. doi: 10.1155/2019/3085756.
4. Lee AH, Dixit VD. Dietary Regulation of Immunity. Immunity. 2020;53:510-523. doi: 10.1016/j.immuni.2020.08.013.
5. Brault C, Schulze A. The Role of Glucose and Lipid Metabolism in Growth and Survival of Cancer Cells. Recent Results Cancer Res. 2016;207:1-22. doi: 10.1007/978-3-319-42118-6_1. PMID: 27557532.
6. Moszak M, Szulińska M, Bogdański P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients. 2020;12:1096. doi: 10.3390/nu12041096.
7. Dedeepiya VD, Sivaraman G, Venkatesh AP, Preethy S, Abraham SJ. Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: preliminary findings from the study on three patients from India. Case Rep Med. 2012;2012:895370.
8. Ganesh JS, Rao YY, Ravikumar R, Jayakrishnan GA, Iwasaki M, Preethy S, et al. Beneficial effects of black yeast derived 1-3, 1-6 Beta Glucan-Nichi Glucan in a dyslipidemic individual of Indian origin–a case report. J Diet Suppl. 2014;11:1-6.
9. Ikewaki N, Fujii N, Onaka T, Ikewaki S, Inoko H. Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement. Microbiol Immunol. 2007;51:861-73.
10. Preethy S, Ikewaki N, Levy GA, Raghavan K, Dedeepiya VD, Yamamoto N, Srinivasan S, Ranganathan N, Iwasaki M, Senthilkumar R, Abraham SJK. Two unique biological response-modifier glucans beneficially regulating gut microbiota and faecal metabolome in a non-alcoholic steatohepatitis animal model, with potential for applications in human health and disease. BMJ Open Gastroenterology 2022;9:e000985. doi: 10.1136/bmjgast-2022-000985
11. Raghavan K, Dedeepiya VD, Yamamoto N, Ikewaki N, Sonoda T, Iwasaki M, Kandaswamy R, Senthilkumar R, Preethy S, Abraham SJK. Benefits of gut microbiota reconstitution by beta 1,3-1,6 glucans in subjects with autism spectrum disorder and other neurodegenerative diseases. Journal of Alzheimer’s Disease.1 Jan. 2022 : 1 – 12. https://doi.org/10.3233/JAD-220388
12. Ikewaki N, Raghavan K, Dedeepiya VD, Suryaprakash V, Iwasaki M, Preethy S, senthilkumar R, Abraham SJK. Beneficial immune-regulatory effects of novel strains of Aureobasidium pullulans AFO-202 and N-163 produced beta glucans in Sprague Dawley rats. Clinical Immunology Communications 2021. https://doi.org/10.1016/j.clicom.2021.11.001
13. Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30.
14. Ikewaki N, Levy GA, Kurosawa G, Iwasaki M, Dedeepiya VD, Vaddi S, Senthilkumar R, Preethy S, Abraham SJK. Hepatoprotective Effects of Aureobasidium pullulans Derived β 1,3-1,6 Glucans in a Murine Model of Non-alcoholic Steatohepatitis. J Clin Exp Hepatol. 2022;12(6):1428-1437. doi: 10.1016/j.jceh.2022.06.008.
15. Ikewaki N, Ikeue Y, Nagataki M, Kurosawa G, Dedeepiya VD, Rajmohan M, Vaddi S, Senthilkumar R, Preethy S, Abraham SJK. Beneficial effects of 1,3-1,6 β-glucans produced by Aureobasidium pullulans on non-esterified fatty acid levels in diabetic KKAy mice and their potential implications in metabolic dysregulation. J Diabetes Metab Disord. 2022;22(1):487-494. doi: 10.1007/s40200-022-01170-5.
16. Ikewaki M, Iwasaki M, Kurosawa G, Rao KS, Beitia JL, Preethy S, et al. β-Glucans: Wide-spectrum Immune-balancing Food-supplement-based Enteric (β-WIFE) Vaccine Adjuvant Approach to COVID-19. Human Vaccines & Immunotherapeutics 2021; 17. doi:10.1080/21645515.2021.1880210.
17. Ikewaki N, Dedeepiya VD, Iwasaki M, Abraham SJK. Commentary: Beyond “TRIM” Benefits of β-Glucan by Blood Glucose and Lipid Balancing Potentials in Its Defense Against COVID-19. Front Immunol. 2021;12:620658. doi: 10.3389/fimmu.2021.620658.
18. Ikewaki N, Iwasaki M, Abraham S. Biological response modifier glucan through balancing of blood glucose may have a prophylactic potential in COVID-19 patients. Journal of Diabetes & Metabolic Disorders 2020. doi: 10.1007/s40200-020-00664-4
19. Ikewaki N, Rao KS, Archibold AD, Iwasaki M, Senthilkumar R, Preethy S, et al. Coagulopathy associated with COVID-19 – Perspectives & Preventive strategies using a Biological Response Modifier Glucan. Thromb J. 2020. doi: 10.1186/s12959-020-00239-6.
20. Raghavan K, Dedeepiya VD, Suryaprakash V, Rao KS, Ikewaki N, Sonoda T, Levy GA, Iwasaki M, Senthilkumar R, Preethy S, Abraham SJ. Beneficial effects of novel aureobasidium pullulans strains produced beta-1,3-1,6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study. Biomed Pharmacother. 2022;145:112243. doi: 10.1016/j.biopha.2021.112243.
21. Preethy S, Raghavan K, Dedeepiya VD, Surya Prakash V, Ikewaki N, Ikeue Y, Nagataki M, Iwasaki M, Senthilkumar R, Abraham SJK. Beneficial Immune Regulation by Biological Response Modifier Glucans in COVID-19 and Their Envisaged Potentials in the Management of Sepsis. Front Immunol. 2022 Jun 27;13:870632.
22. Muramatsu D, Iwai A, Aoki S, Uchiyama H, Kawata K, Nakayama Y, et al.. β-Glucan Derived From Aureobasidium Pullulans is Effective for the Prevention of Influenza in Mice. PloS One (2012) 7(7):e41399. doi: 10.1371/journal.pone.0041399
23. Song, Yonghong & Zhang, Wenzhi & Jaganathan, Ravindran. (2018). Assessment of activity and mechanism of action of β-D-glucan against dengue virus. Tropical Journal of Pharmaceutical Research. 17. 1061-1066. 10.4314/tjpr.v17i6.12.
24. Paris S, Chapat L, Martin-Cagnon N, Durand PY, Piney L, Cariou C, Bergamo P, Bonnet JM, Poulet H, Freyburger L, De Luca K. β-Glucan as Trained Immunity-Based Adjuvants for Rabies Vaccines in Dogs. Front Immunol. 2020 Oct 8;11:564497.
25. Kim CJ, Park J, Kang SW. Prevalence of metabolic syndrome and cardiovascular risk level in a vulnerable population. Int J Nurs Pract. 2015;21:175-83. doi: 10.1111/ijn.12258.
26. UMIN-CTR Clinical Trial. Exploratory trial of clinical effects by ingestion of beta-glucan-containing test-food https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000046681 (Accessed 04Aug21).
27. Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z.
28. Xiao E, Luo L. Alternative Therapies for Diabetes: A Comparison of Western and Traditional Chinese Medicine (TCM) Approaches. Curr Diabetes Rev. 2018;14:487-496. doi: 10.2174/1573399813666170519103230.
29. Ott C, Schmieder RE. The role of statins in the treatment of the metabolic syndrome. Curr Hypertens Rep. 2009;11(2):143-9.
30. Gostinčar C, Turk M, Zajc J, Gunde-Cimerman N. Fifty Aureobasidium pullulans genomes reveal a recombining polyextremotolerant generalist. Environ Microbiol. 2019;21:3638-3652. doi: 10.1111/1462-2920.14693.
31. Jain S, Gautam V, Naseem S. Acute-phase proteins: As diagnostic tool. J Pharm Bioallied Sci. 2011;3:118-27. doi: 10.4103/0975-7406.76489.
32. Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019;11:eaau9240. doi: 10.1126/scitranslmed.aau9240.
33. Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021 ;39:101061. doi: 10.1016/j.eclinm.2021.101061.
34. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398:599-607.
35. Murphy EJ, Rezoagli E, Major I, Rowan NJ, Laffey JG. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J Fungi (Basel). 2020 Dec 10;6(4):356
36. Dhewantara F.X. Kusmiati Cholesterol-Lowering Effect of Beta Glucan Extracted from Saccharomyces cerevisiae in Rats. Sci. Pharm. 2016;84:153–165. doi: 10.3797/scipharm.isp.2015.07.
37. Bashir K.M.I., Choi J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017;18:1906. doi: 10.3390/ijms18091906.
38. Jayachandran M., Chen J., Chung S.S.M., Xu B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018;61:101–110. doi: 10.1016/j.jnutbio.2018.06.010.
39. Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408
40. Smeekens S.P., Gresnigt M.S., Becker K.L., Cheng S.-C., Netea S.A., Jacobs L., Jansen T., Van De Veerdonk F.L., Williams D.L., Joosten L.A., et al. An anti-inflammatory property of Candida albicans β-glucan: Induction of high levels of interleukin-1 receptor antagonist via a Dectin-1/CR3 independent mechanism. Cytokine. 2015;71:215–222. doi: 10.1016/j.cyto.2014.10.013
41. Moorlag SJCFM, Khan N, Novakovic B, Kaufmann E, Jansen T, van Crevel R, Divangahi M, Netea MG. β-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1. Cell Rep. 2020 May 19;31(7):107634.
42. Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009 Jun 10;2:25. doi: 10.1186/1756-8722-2-25.
43. He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:73.
44. Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2020;136:104714. doi: 10.1016/j.nbd.2019.104714.
45. Okumura T. NK cell cytotoxicity in different age groups of healthy volunteers. Gendai kagaku (Chemistry today) 1984: 11: 40.
46. Mio M. Effect of oral intake of black yeast beta-glucan on NK activity in the elderly and patients with cancer. Abstract presented at 29th Annual Meeting of the Japanese Society of Venous and Enteral Nutrition Pacifico Yokohama, Japan 2014.
© The Authors 2023