F. Bellelli1,2
1. Fellowship in Geriatric and Gerontology, University of Milan, Milan, Italy; 2. Gérontopôle de Toulouse, Institut du Vieillissement, Centre Hospitalo-Universitaire de Toulouse, Toulouse, France.
Corresponding Author: Federico Bellelli, MD, Fellowship in Geriatric and Gerontology, University of Milan, Milan, Italy, + 39 3319709061, federico.bellelli@unimi.it
J Aging Res & Lifestyle 2024;13:60-64
Published online May 22, 2024, http://dx.doi.org/10.14283/jarlife.2024.8
Abstract
Recent findings suggest that brain-stimulating activities may have beneficial effects on both Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD). However, whether cognitive interventions merely enhance cognitive reserve or truly attenuate, or even reverse, the disease’s pathophysiology is still controversial. The aim of the present article is to discuss the potential for brain-stimulating activities, including cognitive stimulation (CS), cognitive rehabilitation (CR), and cognitive training (CT), to be symptomatic or disease-modifying interventions in the context of cognitive decline. While emerging evidence indicates that CT can enhance synaptic plasticity, suggesting a potential role in augmenting cognitive reserve, its impact on AD pathology remains uncertain. Small-scale studies suggest that CT and CS may slow down neurodegeneration in MCI patients and that multidomain interventions combining physical activity with CT may benefit Aβ pathology. However, the considerable heterogeneity across studies limits the comparability of findings. It underscores the necessity for a more standardized approach to cognitive interventions in future guidelines for preventing and managing cognitive decline.
Key words: Cognitive stimulation, cognitive rehabilitation, cognitive training, Alzheimer disease, disease-modifying treatments.
The World Health Organization (WHO) and the National Institute for Health and Care Excellence (NICE) recommend social interactions and other brain-stimulating activities as non-pharmacological treatments for dementia (1, 2). However, the extent to which such interventions merely alleviate signs and symptoms of cognitive decline, reduce the pathophysiological burden of Alzheimer’s disease (AD – most prevalent type of dementia), benefit both aspects or have no discernible effect remains unclear and deserve further debate. The present article aims to discuss the potential for brain-stimulating activities to be symptomatic or disease-modifying interventions in the context of cognitive decline during aging.
Brain stimulating activities
Among various brain-stimulating interventions, most recommendations endorse cognitive stimulation (CS) (1, 3), cognitive rehabilitation (CR)(1), or cognitive training (CT) (4). CS consists of various activities and discussions aimed at improving social and cognitive functioning (1). Similarly, CR works to achieve goals relevant to the person living with dementia and his family, trying to enhance and maintain functioning in everyday life (1). On the other hand, CT is a more specific approach that works on a set of standardized tasks designed to reflect singular cognitive functions (i.e., episodic memory) (1) and is, therefore, particularly suitable for individuals with Mild Cognitive Impairment (MCI) (5).
Multiple systematic reviews have suggested in the last two decades that individuals with MCI may experience slightly to moderate improvements following cognitive interventions. Still, the heterogeneity of the studies made it challenging to distinguish which method had the strongest impact (6, 7). Recently, a systematic review and meta-analysis extended the evaluation of cognitive interventions to individuals with AD dementia. Analyzing 25 studies involving 2012 participants, the review concluded that while further research on CR and CS is warranted, there is some indication of temporary benefits on global cognitive function following CT (8).
However, the mechanisms by which these interventions may improve cognitive functions are still debated. Indeed, according to the cognitive reserve hypothesis, individuals with a greater cognitive reserve can withstand a higher AD pathological burden before developing dementia by employing mental processing approaches or compensatory brain networks (synaptic plasticity) (9). Given that, do cognitive interventions enhance cognitive reserve, or do they genuinely attenuate or reverse the disease’s pathophysiology?
Tackling cognitive decline symptoms through improved cognitive reserve
Recent findings suggest that cognitive interventions might have a beneficial impact on synaptic plasticity and, consequently, on cognitive reserve. Indeed, several studies have demonstrated that CT can enhance regional activity in functional Magnetic Resonance Imaging (fMRI) scans of patients with MCI following an intervention ranging from 2 weeks to 12 months (10–13). In particular, Hampstead et al. proposed that the most robust training-specific increases occur within areas of the default network that are abnormal in MCI and AD (medial frontal and parietal cortices and around the temporoparietal junction) (12). Accordingly, two studies found that compared to standard care, 8-week CR and 7-week CS programs could improve fMRI even in individuals with mild dementia (14, 15). Interestingly, Bentham et al. observed that individuals with high vascular burden had a lower functional connectivity response to CS than those with low burden, suggesting that vascular pathology could limit the potential for a neuroplastic response to cognitive interventions (16).
Brain stimulation and the biomarkers of AD
Regarding the core biomarkers of AD (Aβ and Tau), evidence on cognitive interventions remains scarce and inconclusive. A small study employed a questionnaire to retrospectively evaluate the engagement in cognitively stimulating activities (e.g., reading, writing, playing games) across the lifespan of 65 healthy older adults and 10 AD patients. The study observed greater participation in cognitively stimulating activities, particularly during early and middle adulthood, was associated with decreased amyloid PET burden compared to individuals with limited involvement in such activities (17). Accordingly, secondary analyses of a subset of the Multidomain Alzheimer Preventive Trial (MAPT), an extensive study on community-dwelling older adults at risk of cognitive decline, suggested that a three-year multidomain intervention, including CT and physical activity (PA) advice, was associated with lower Aβ burden on amyloid PET compared to controls (18). In contrast, no significant difference was observed in plasma phosphorylated-tau levels (19). However, a small study on community-dwelling older adults (n=27) suggested that PA rather than CT may primarily drive the effects on Aβ levels. Indeed, the study reported reduced plasma Aβ levels after a 12-week program in both single-task (PA) and dual-task training groups (PA + CT), with no between-group difference (20).
On the other hand, a similar study on individuals with AD (n=34) observed that an 8-week dual-task training significantly lowered plasma Aβ levels. In contrast, no significant difference was reported for the group doing only PA, suggesting that CT might influence AD pathophysiology (21). Notably, a study on individuals with MCI assessing the effects of a 9-month cognitive intervention alone (mindful awareness practice; MAP) on Aβ-42 levels (salivary sample) found no significant differences between the treatment arm (MAP) and the active control group (22). Even in preclinical AD models, evidence remains unclear, with some studies suggesting decreased (23-25), no change (26, 27), or increased (28) Aβ load in transgenic mice exposed to cognitive stimulation (i.e., enriched environment or spatial training).
Brain stimulation and the biomarkers of neurodegeneration
According to the Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease, anatomic MRI and Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) are biomarkers of non-specific degeneration in AD pathophysiology (29). However, cognitive interventions’ effects on structural and metabolic changes in the brain are still unclear. A retrospective study involving 329 cognitively unimpaired middle-aged adults revealed that individuals engaging in cognitively stimulating activities, such as playing card games, exhibited larger gray matter volumes in brain regions susceptible to AD pathology (30). In contrast, secondary analysis of a subset (n= 244) of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large-scale trial involving older adults at risk for dementia, found no changes in regional brain volumes or cortical thickness after two years of a multidomain intervention comprising PA, CT, diet, and vascular risk monitoring (31). The study suggests that multidomain intervention has no beneficial effect on neurodegeneration among cognitively unimpaired individuals at risk of AD. However, the design of the FINGER study did not permit the evaluation of the impact of interventions in individuals already experiencing some level of cognitive impairment. Three small-scale studies suggested that cognitive interventions may benefit MRI changes in individuals with MCI. Specifically, a small study allocated individuals with mild dementia to either a 7-week CS program (n=16) or standard care (n=13). The study observed that individuals in the treatment group maintained their total brain volume, as assessed by MRI, while those in the standard care group experienced a decrease (15). Accordingly, another study suggested that a 6-month multi-intervention program based on aerobic exercises and CS could decelerate atrophy in AD-related brain regions among MCI patients (32). Furthermore, the last study observed that undergoing 24 sessions of computerized CT led to focal increases in cortical thickness among individuals with MCI (33), even hinting at a potential for reversibility of neurodegeneration.
Regarding brain metabolism, some studies showed a trend toward regional metabolic changes following cognitive interventions among cognitively unimpaired individuals at risk for dementia. Secondary analyses of a subset population (n=67) of the MAPT study suggested that combined treatment with omega-3 supplementation and multidomain intervention (CT and physical activity; PA) did not significantly increase overall brain metabolism after six or twelve months of treatment. However, exploratory analyses employing voxel-wise approaches suggested that multidomain intervention could enhance metabolism in specific brain regions, including the right hippocampus, right posterior cingulate, left posterior para-hippocampal gyrus, and right insular cortex (34). Likewise, a small study on community-dwelling older adults (n=45) showed that a 16-week computerized CT had a trend toward a metabolic increase in the right inferior frontal gyrus without reaching the statistical significance (35). However, evidence in individuals with cognitive decline is limited and contradicting. A small study suggested that a six-month cognitive intervention might decelerate the widespread cortical metabolic decline in AD and MCI patients, with a more pronounced effect observed in the latter (36). On the contrary, another study observed that a 12-week home-based CT program did not yield significant improvements in brain metabolism in individuals with MCI (37).
Conclusions
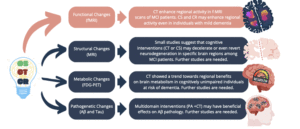
In conclusion, evidence indicates a rise in synaptic plasticity following CT, suggesting that the beneficial effects of cognitive interventions may be partially attributed to the enhancement of cognitive reserve. However, whether cognitive interventions can attenuate or reverse AD pathophysiology (amyloid beta, tau, and neurodegeneration) remains subject to debate, and further studies are required (Figure 1). The need for robust evidence in this field could be attributed to various factors, including small study sample sizes, the substantial methodological heterogeneity across trials (rendering comparability across studies a problematic exercise), and combined interventions. Indeed, most studies evaluated the combined effects of physical activity and CT, making it challenging to discern the effects of one intervention. Additionally, the high cost of neuroimaging and limited accessibility to AD core biomarkers in cerebrospinal fluid (CSF) have posed significant constraints on large-scale population studies in previous years. However, the recent validation of more cost-effective and less invasive plasma-based biomarkers (29) may offer a valuable opportunity further to investigate cognitive interventions’ effects on AD pathogenesis.
Legend: CS, Cognitive Stimulation; CT, Cognitive Training; CR, Cognitive Rehabilitation; fMRI, functional Magnetic Resonance Imaging; FDG-PET, Fluorodeoxyglucose Positron Emission Tomography; MCI, Mild Cognitive Impairment; PA, Physical Activity
Moreover, considering that cognitive interventions may enhance cognitive reserve, it is reasonable to expect different results based on participants’ baseline cognitive reserve. Therefore, further studies must consider at least the subjects’ educational level. Lastly, the primary reason for more evidence in the field might be the substantial heterogeneity of the studies. Notably, the duration (ranging from a few days to 3 years) and the methodologies employed in cognitive interventions were quite different between various trials. Indeed, although most guidelines endorse non-pharmacological approaches as first-line treatment for dementia (4) and MCI (38), official recommendations regarding the specifics of these interventions have often been lacking in previous years. Recently, the WHO ICOPE guidelines recommended cognitive stimulation for older adults with cognitive impairment, suggesting a standard group approach involving up to 14 themed sessions lasting approximately 45 minutes each, held twice a week (3). Continuing this path, future guidelines on the prevention of cognitive decline, as well as on the treatment and management of dementia, should aim to standardize at least the duration and the modalities of cognitive interventions while preserving the person-centered care approach that is a cornerstone of geriatric medicine.
Conflict of Interest: The author declares no conflicts of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Dementia: assessment, management and support for people living with dementia and their carers. NICE Guideline. 2018. https://www.nice.org.uk/guidance/ng97. Accessed 21 Feb 2024.
2. World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization. 2019. https://iris.who.int/handle/10665/312180. Accessed 21 Feb 2024.
3. Integrated care for older people (ICOPE): guidance for person-centred assessment and pathways in primary care. 2019. https://www.who.int/publications-detail-redirect/WHO-FWC-ALC-19.1. Accessed 21 Feb 2024.
4. Kane RL, Butler M, Fink HA, et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017. http://www.ncbi.nlm.nih.gov/books/NBK442425/ Accessed 22 Feb 2024.
5. Zhang H, Huntley J, Bhome R, et al. Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: a systematic review and meta-analysis. BMJ Open. 2019 Aug 1;9(8):e027062. DOI: 10.1136/bmjopen-2018-027062
6. Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res Rev. 2013 Jan 1;12(1):263–75. DOI: 10.1016/j.arr.2012.07.003
7. Simon SS, Yokomizo JE, Bottino CMC. Cognitive intervention in amnestic Mild Cognitive Impairment: A systematic review. Neurosci Biobehav Rev. 2012 Apr 1;36(4):1163–78. DOI: 0.1016/j.neubiorev.2012.01.007
8. Wang YY, Yang L, Zhang J, Zeng XT, Wang Y, Jin YH. The Effect of Cognitive Intervention on Cognitive Function in Older Adults With Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuropsychol Rev. 2022 Jun 1;32(2):247–73. DOI: 10.1007/s11065-021-09486-4
9. Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer Disease and Cognitive Reserve: Variation of Education Effect With Carbon 11–Labeled Pittsburgh Compound B Uptake. Arch Neurol. 2008 Nov 10;65(11):1467–71. DOI: 10.1001/archneur.65.11.1467
10. Rosen AC, Sugiura L, Kramer JH, Whitfield-Gabrieli S, Gabrieli JD. Cognitive Training Changes Hippocampal Function in Mild Cognitive Impairment: A Pilot Study. J Alzheimers Dis. 2011;26(Suppl 3):349–57. DOI: 10.3233/JAD-2011-0009
11. Li B, Tang H, He G, et al. Tai Chi enhances cognitive training effects on delaying cognitive decline in mild cognitive impairment. Alzheimers Dement. 2023;19(1):136–49. DOI: 10.1002/alz.12658
12. Hampstead BM, Stringer AY, Stilla RF, et al. ACTIVATION AND EFFECTIVE CONNECTIVITY CHANGES FOLLOWING EXPLICIT MEMORY TRAINING FOR FACE-NAME PAIRS IN PATIENTS WITH MILD COGNITIVE IMPAIRMENT: A PILOT STUDY. Neurorehabil Neural Repair. 2011;25(3):210–22. DOI: 10.1177/1545968310382424
13. Belleville S, Clément F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain J Neurol. 2011 Jun;134(Pt 6):1623–34. DOI: 10.1093/brain/awr037
14. van Paasschen J, Clare L, Yuen KSL, Woods RT, Evans SJ, Parkinson CH, et al. Cognitive rehabilitation changes memory-related brain activity in people with Alzheimer disease. Neurorehabil Neural Repair. 2013 Jun;27(5):448–59. DOI: 10.1177/1545968312471902
15. Liu T, Spector A, Mograbi DC, Cheung G, Wong GHY. Changes in Default Mode Network Connectivity in Resting-State fMRI in People with Mild Dementia Receiving Cognitive Stimulation Therapy. Brain Sci. 2021 Sep;11(9):1137. DOI: 10.3390/brainsci11091137
16. Bentham C, De Marco M, Venneri A. The Modulatory Effect of Cerebrovascular Burden in Response to Cognitive Stimulation in Healthy Ageing and Mild Cognitive Impairment. Neural Plast. 2019 Aug 6;2019:2305318. DOI: 10.1155/2019/2305318
17. Landau SM, Marks SM, Mormino EC, et al. Association of Lifetime Cognitive Engagement and Low β-Amyloid Deposition. Arch Neurol. 2012 May 1;69(5):623–9. DOI: 10.1001/archneurol.2011.2748
18. Hooper C, Coley N, De Souto Barreto P, et al. Cortical β-Amyloid in Older Adults Is Associated with Multidomain Interventions with and without Omega 3 Polyunsaturated Fatty Acid Supplementation. J Prev Alzheimers Dis. 2020 Mar 1;7(2):128–34. DOI: 10.14283/jpad.2020.4
19. Coley N, Zetterberg H, Cantet C, et al. Plasma p-tau181 as an outcome and predictor of multidomain intervention effects: a secondary analysis of a randomised, controlled, dementia prevention trial. Lancet Healthy Longev. 2024 Feb;5(2):e120–30. DOI: 10.1016/S2666-7568(23)00255-6
20. Yokoyama H, Okazaki K, Imai D, et al. The effect of cognitive-motor dual-task training on cognitive function and plasma amyloid β peptide 42/40 ratio in healthy elderly persons: a randomized controlled trial. BMC Geriatr. 2015 May 28;15:60. DOI: 10.1186/s12877-015-0058-4
21. Nam SM, Kim S gil. Dual-Task Training Effect on Cognitive and Body Function, β-amyloid Levels in Alzheimer’s Dementia Patients: A Randomized Controlled Trial. J Korean Phys Ther. 2021 Jun 30;33(3):136–41. DOI: 10.18857/jkpt.2021.33.3.136
22. Ng TKS, Slowey PD, Beltran D, Ho RCM, Kua EH, Mahendran R. Effect of mindfulness intervention versus health education program on salivary Aβ-42 levels in community-dwelling older adults with mild cognitive impairment: A randomized controlled trial. J Psychiatr Res. 2021 Apr 1;136:619–25. DOI: 10.1016/j.jpsychires.2020.10.038
23. Herring A, Lewejohann L, Panzer AL, Donath A, Kröll O, Sachser N, et al. Preventive and therapeutic types of environmental enrichment counteract beta amyloid pathology by different molecular mechanisms. Neurobiol Dis. 2011 Jun;42(3):530–8. DOI: 10.1016/j.nbd.2011.03.007
24. Gerenu G, Dobarro M, Ramirez MJ, Gil-Bea FJ. Early cognitive stimulation compensates for memory and pathological changes in Tg2576 mice. Biochim Biophys Acta BBA – Mol Basis Dis. 2013 Jun 1;1832(6):837–47. DOI: 10.1016/j.bbadis.2013.02.018
25. Mehla J, Deibel SH, Karem H, et al. Repeated multi-domain cognitive training prevents cognitive decline, anxiety and amyloid pathology found in a mouse model of Alzheimer disease. Commun Biol. 2023 Nov 10;6:1145. DOI: 10.1038/s42003-023-05506-6
26. Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004 Aug 6;15(11):1751–4. DOI: 10.1097/01.wnr.0000137183.68847.4e
27. Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, et al. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007 Jun;28(6):831–44. DOI: 10.1016/j.neurobiolaging.2006.04.009
28. Stuart KE, King AE, Fernandez-Martos CM, Summers MJ, Vickers JC. Environmental novelty exacerbates stress hormones and Aβ pathology in an Alzheimer’s model. Sci Rep. 2017 Jun 5;7:2764. DOI: 10.1038/s41598-017-03016-0
29. AAIC. Revised Criteria for Diagnosis and Staging of Alzheimer’s. 2023. http: //aaic.alz.org/diagnostic-criteria.asp. Accessed: 24 Feb 2024.
30. Schultz S, Larson J, Oh J, et al. Participation in cognitively-stimulating activities is associated with brain structure and cognitive function in preclinical Alzheimer’s disease. Brain Imaging Behav. 2015 Dec;9(4):729–36. DOI: 10.1007/s11682-014-9329-5
31. Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Res Ther. 2019 Jun 4;11(1):53. DOI: 10.1186/s13195-019-0506-z
32. Köbe T, Witte AV, Schnelle A, et al. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. NeuroImage. 2016 May 1;131:226–38. DOI: 10.1016/j.neuroimage.2015.09.050
33. Na HR, Lim JS, Kim WJ, et al. Multimodal Assessment of Neural Substrates in Computerized Cognitive Training: A Preliminary Study. J Clin Neurol Seoul Korea. 2018 Oct;14(4):454–63. DOI: 10.3988/jcn.2018.14.4.454
34. Delrieu J, Voisin T, Saint-Aubert L, et al. The impact of a multi-domain intervention on cerebral glucose metabolism: analysis from the randomized ancillary FDG PET MAPT trial. Alzheimers Res Ther. 2020 Oct 19;12(1):134. DOI: 10.1186/s13195-020-00683-6
35. Shah T, Verdile G, Sohrabi H, et al. A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. Transl Psychiatry. 2014 Dec 2;4(12):e487. DOI: 10.1038/tp.2014.122
36. Förster S, Buschert VC, Teipel SJ, et al. Effects of a 6-month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer’s disease. J Alzheimers Dis JAD. 2011;26 Suppl 3:337–48. DOI: 10.3233/JAD-2011-0025
37. Park J, Kim SE, Kim EJ, et al. Effect of 12-week home-based cognitive training on cognitive function and brain metabolism in patients with amnestic mild cognitive impairment. Clin Interv Aging. 2019 Jun 28;14:1167–75. DOI: 10.2147/CIA.S200269
38. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment. Neurology. 2018 Jan 16;90(3):126–35. DOI: 10.1212/WNL.0000000000004826
© The Authors 2024